The reason for replacing graphite in the electrodes of the ubiquitous lithium-ion (Li-ion) battery is clear to anyone who uses a smartphone: The batteries run out of charge in just a few hours under regular use.
One answer has been to replace the graphite with silicon. Unfortunately, the expanding and contracting that occurred as the lithium ions transported in and out of silicon electrodes quickly cracks it.
The next solution was to create “nanostructured silicon” electrodes, sometimes with the help of graphene or good old carbon nanotubes.
Now researchers at the University of California San Diego (UCSD) have brought a new perspective to the issue. They are taking a page from band-gap engineering, in which heterostructures are used to create energy barriers between electrons and holes, and applied the concept to creating barriers to the ions as they enter into an electrode so they diffuse in a very specific way.
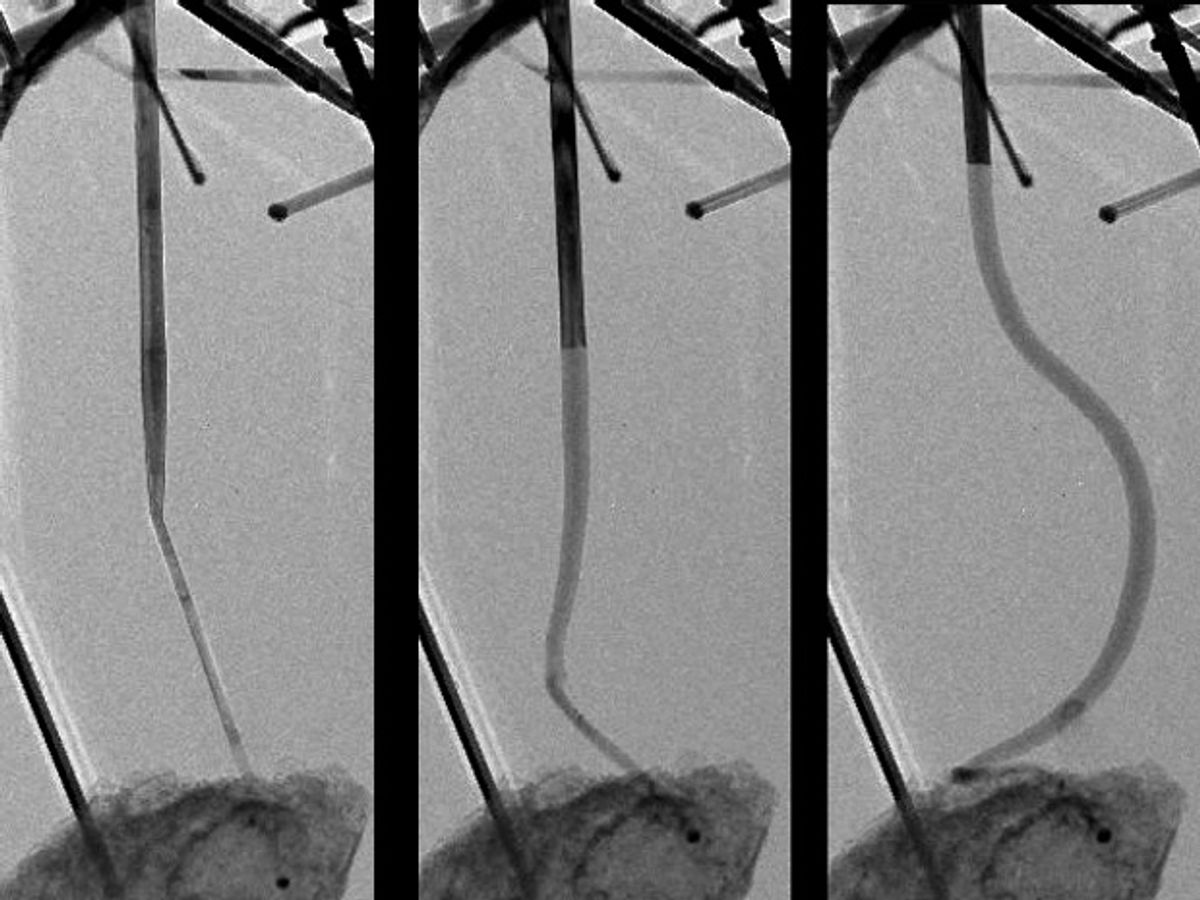
The research, which was published in the journal Nano Letters (“Tailoring Lithiation Behavior by Interface and Bandgap Engineering at the Nanoscale”), describes a method by which the typical surface diffusion of lithium ions into a nanowire electrode is blocked and instead the ions are diffused layer-by-layer along the length of the nanowire.
As the Nano Letters article notes: “These results demonstrate for the first time that interface and band-gap engineering of electrochemical reactions can be utilized to control the nanoscale ionic transport / insertion paths and thus may be a new tool to define the electrochemical reactions in Li-ion batteries.”
In the video below you can see the different way this impacts the nanowire. Instead of blowing up around its middle, it gradually grows along its axis as the lithium ions transport into the nanowire. The video also demonstrates some new breakthroughs in nanoscale imaging with transmission electron microscopy. It shows lithium-ion reactions in real time at nanoscale precision.
In the press release about his work, Shadi Dayeh, a professor at UCSD, explains that this control of how the ions diffuse could result in “an effective way to tailor volume expansion of lithium ion battery electrodes, which could potentially minimize their cracking, improve their durability, and perhaps influence how one could think about different electrode architectures.”
The new technology starts with germanium nanowires that are then coated with silicon.The research builds on previous work of Dayeh and his colleagues published in the journal Applied Physics Letters and again in Nano Letters in which they demonstrated control over the heterostructuring of germanium-silicon nanowires.
According to Dayeh, the new electrodes would allow for battery designs in which the expansion of the electrodes would not cause any shorting between the cathode and the anode.
Images: UC San Diego Electrical and Computer Engineering
Dexter Johnson is a contributing editor at IEEE Spectrum, with a focus on nanotechnology.