The Practical Power of Fusing Photons
Changing the color of light supercharges solar energy, 3D printing, and night vision

Color plays a huge role in most of our lives. It signals danger or caution, like the patterns on a poisonous frog or the color of a stoplight. It evokes joy and inspiration through nature, art, and fashion. It can even trigger the recollection of a favorite memory, through pictures of family and friends.
In the technical world, the color of light is no less important. It affects the efficiency of solar cells, how far we can see inside our bodies, and the speed of 3-D printing.
But light can be useful only if you can actually get it where it needs to go; many materials will absorb or scatter the light long before it can reach its intended destination.
We in the Congreve Lab at Stanford University became interested in changing the color of light for exactly this reason: It can help us get the right kind of light to the right place.
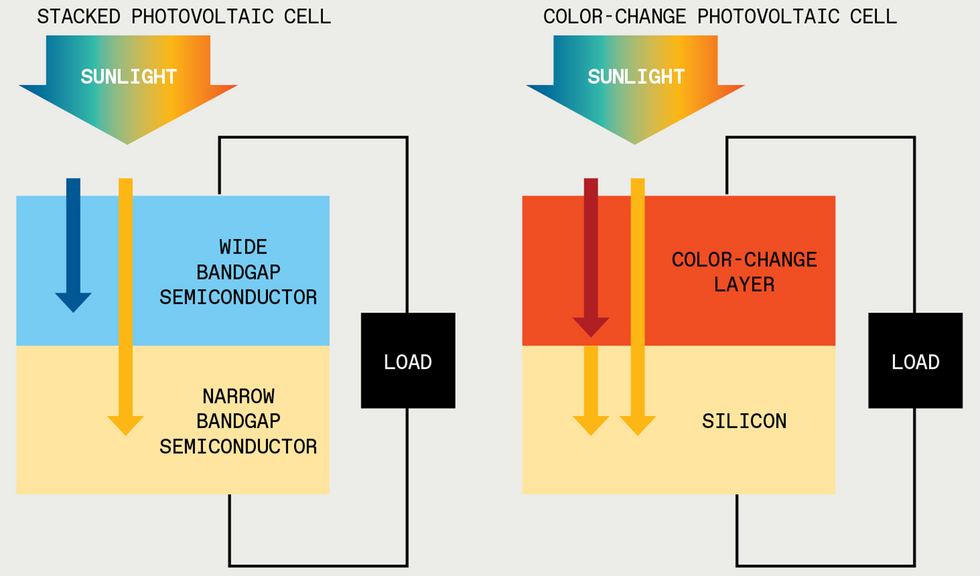
Initially, we focused on developing color-changing technology for use in solar energy, where its usefulness is obvious. Photovoltaic cells harvest energy from only a limited range of energies—that is, colors. That range differs depending on the material used to produce the solar cell, but it’s always limited. One approach to improving solar-cell efficiency has been to produce cells with multiple layers tuned to different energy levels. But there is another way to think about it: It could be simpler and more efficient to change the light to fit the cell.
The Process of Upconversion
Before we tell you more about how this process can boost solar power as well as revolutionize 3D printing and enable some other exciting applications, we’ll explain how this technology works.
Traditionally, the color of a photon (defined by its energy or wavelength) is a given. Yet it turns out that we can turn two low-energy photons into a single higher-energy photon using a process called upconversion.
Upconversion has been observed in experiments for more than 50 years, but low efficiencies kept it a laboratory curiosity until materials that more efficiently emit upconverted light were discovered. Even then, issues with extracting a practical number of upconverted photons, how to incorporate the substances into the solid materials needed for real-life applications, and the availability of workable wavelengths have blocked upconversion’s path to commercialization.
Lately, however, a flurry of effort from scientists around the world has led to substantial advances in each of those challenging areas. Most notably, researchers have discovered new materials, made up of inorganic nanoparticles and metal-organic compounds, to increase the range of input and output wavelengths.
In our lab, we use these materials to perform a type of upconversion process called triplet-triplet annihilation, which we’ll explain in a moment. There are other approaches that use the intrinsic abilities of some rare heavy elements to conduct upconversion. But we chose the triplet-triplet annihilation path because the input energies required are low, so we don’t need expensive pulsed lasers. Instead, we can use low-power lasers or even light-emitting diodes, whose intensity is similar to that of laser pointers. Just as important, the materials we are using are more abundant. Together, these characteristics put our technology on an easier path to commercialization.
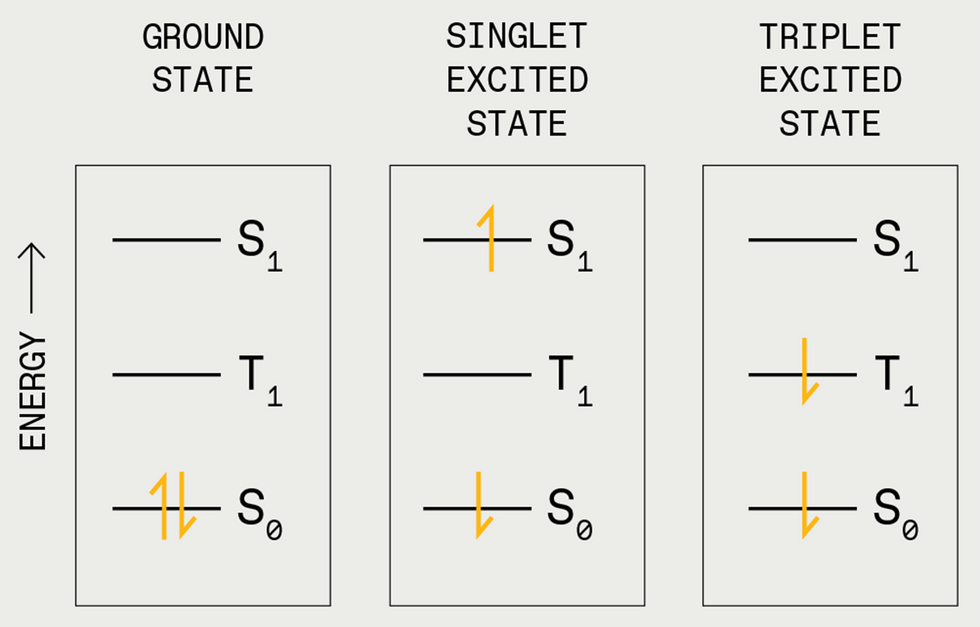
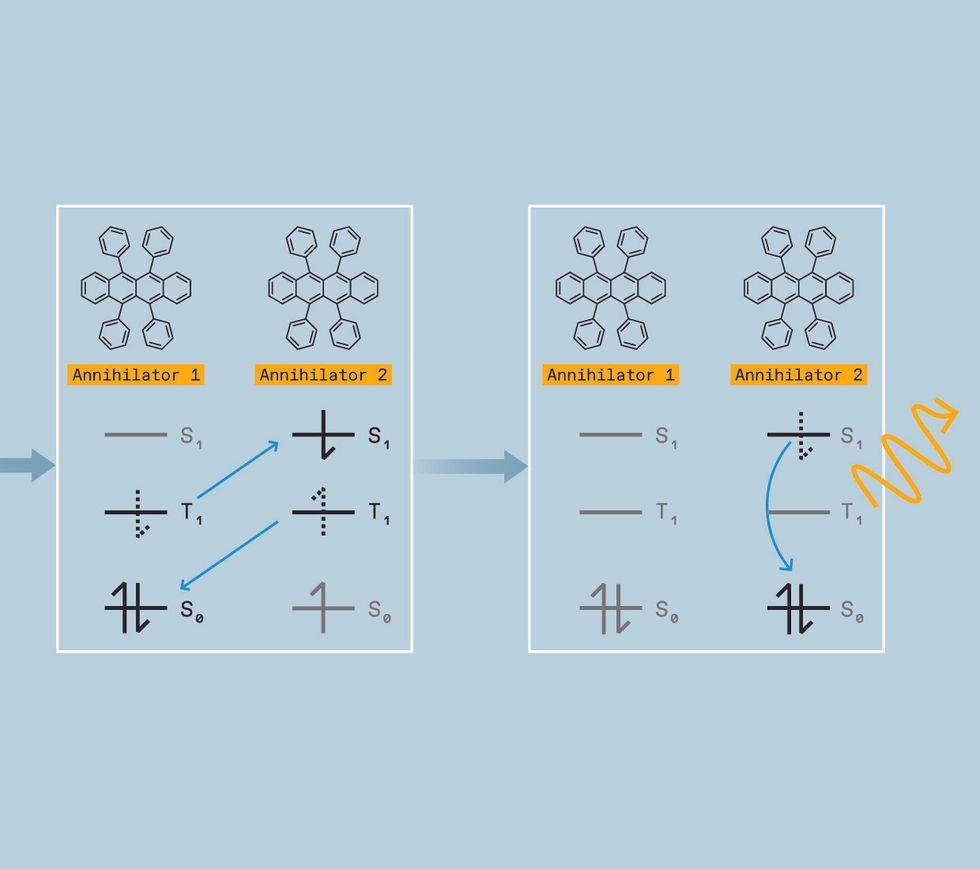
To understand how triplet-triplet annihilation works, you first need to grasp the concept of electron spin states in organic molecules. Electrons in a molecule are organized in discrete locations. Think of the molecule as a multistory house. Each floor has a single room representing one of these locations, or molecular orbitals. Each room can hold two electrons, but they don’t make for good roommates unless the electrons possess opposite qualities called spin states. Electrons first fill the bottom floors—the lowest energy locations—until all electrons have a room. If a photon hits this molecule (the house), it can excite one of the electrons to a higher energy state, pushing it to an unoccupied room on a higher floor. The electron remains there only for a couple nanoseconds or so before it falls back to the ground state—that is, its original room.
When an electron moves back to the ground state, the molecule releases the same amount of energy—in the form of heat or light—that it absorbed to excite the electron. The short-lived excited state, in which the electron is up in its higher orbital, is called a singlet exciton.
There are other types of excitons. For example, there is a state in which the spins are unpaired (both spin up). Here, once one of the electrons is kicked up to a higher room, it cannot easily relax back to its ground state because that room is already occupied by an electron with the same spin. Still, it does eventually get there. Paint, stickers, and toys that glow in the dark after a period of exposure to light exploit this time lag.
Apart from its use in novelty products, this species, called a triplet exciton, is usually viewed as a nuisance. For instance, in organic light-emitting diodes (OLEDs), it’s the singlet excited states that emit light. But both singlet and triplet excited states form in an OLED, with the triplet states reducing the light we see and generating excess heat, both things you don’t want in a display technology.
When you’re trying to manipulate the color of light, however, these triplet states have a silver lining. If two molecules in triplet states collide, they can sometimes combine their energy. This process is called triplet-triplet annihilation.
What interests us is that after combining, the resulting molecule can emit a photon at a shorter wavelength—with higher energy—just as if the molecule had been excited with higher-energy light directly. Here’s how we make that happen.
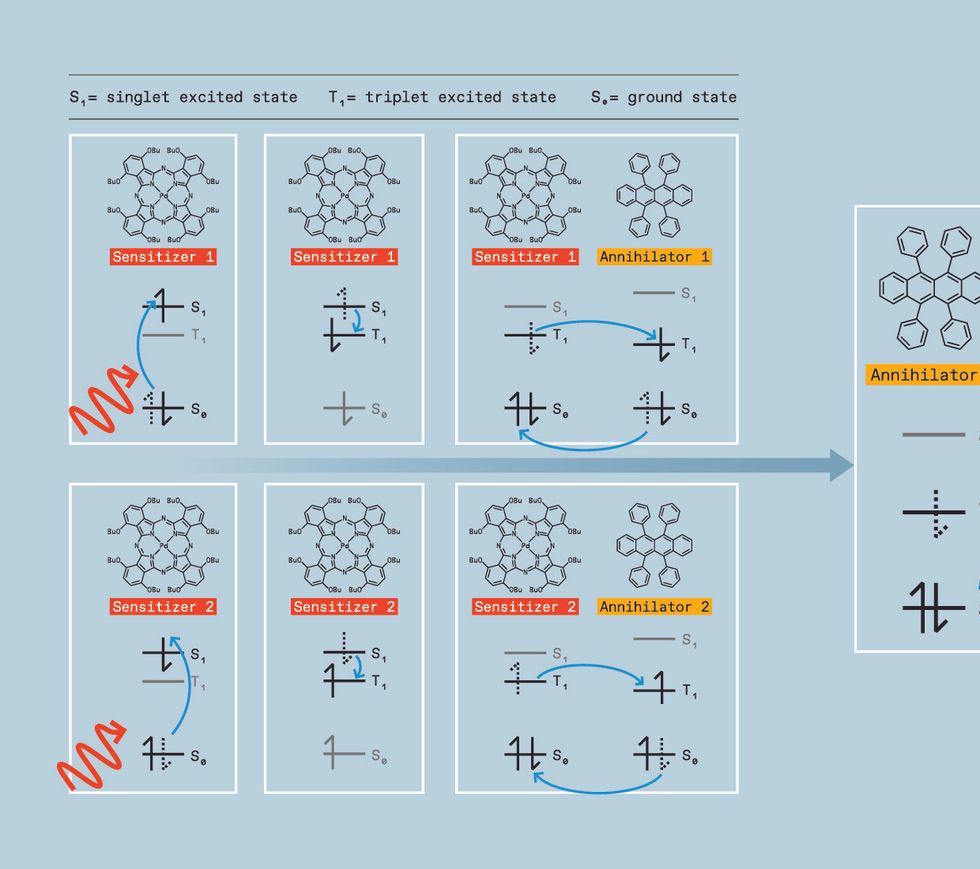
We start by generating the triplet excited state, which is a challenge. While several classes of molecules, called organic semiconductor annihilators, can allow triplets to combine, they don’t form triplets themselves when directly hit with light. Instead, we need to use a material called a triplet sensitizer. Triplet sensitizers typically contain a heavy metal like palladium, iridium, or platinum. This heavy metal serves as a mediator, creating a path for the molecule to move a singlet excited state to a lower-energy triplet excited state instead of falling directly to the ground state.
The sensitizer can then donate its triplet to an annihilator molecule, which possesses a triplet excited state slightly lower in energy than the sensitizer’s. When its energy is transferred to the annihilator, the sensitizer returns to its ground state without releasing light. The annihilator molecule will eventually emit light—but not just yet.
To get the energy released as light, we need two annihilators in the triplet excited state. So we keep pumping low-energy light into the sensitizers, allowing them to repeat this process over and over, generating multiple excited annihilators and increasing the chances of two of these excited annihilators colliding.
When such a mash-up happens, the annihilators transfer energy in a process called triplet-triplet annihilation, converting one molecule into the singlet excited state and the other molecule into the ground state.
That singlet, however, has the combined energy of two triplets. So when it relaxes into the ground state, the photon it emits is higher in energy than the original photon absorbed by the sensitizer. We have upconverted two low-energy photons into one-high energy photon. In terms of colors, that means we can take two red photons and turn them into a blue one, for example, or take two infrared photons and turn them into a yellow one.
Why Color Matters in Photovoltaics
And that’s how we change the color of light. Now to get back to the reason we started doing this: photovoltaics.
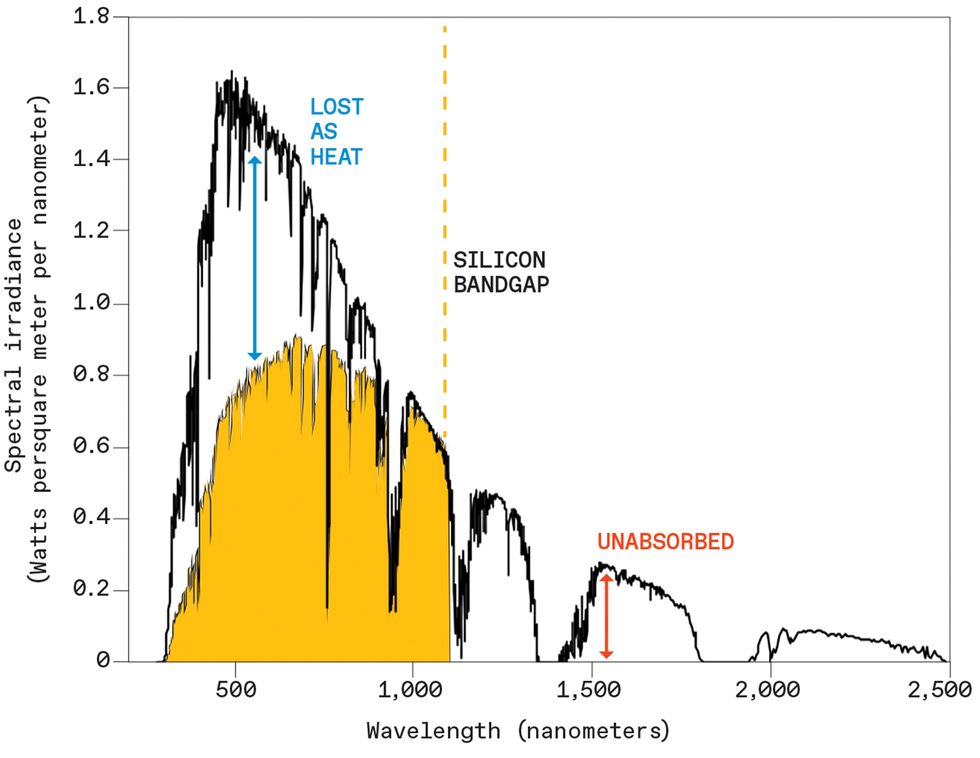
Sunlight offers abundant photons spanning a wide range of energies, from the ultraviolet through the visible spectrum and into the infrared. Yet we use only a fraction of the available photons. That’s why a typical single-junction solar cell—a cell made of one layer of light-absorbing material—has a theoretical efficiency limit of just 34 percent; typical commercial solar cells today are only 15 to 20 percent efficient. The single largest source of this loss is a mismatch between the colors of incoming light and the colors of light that can be used by a solar cell.
To understand this situation, remember that photovoltaic cells are made of semiconductors, materials that possess an energy bandgap. When energy is applied, electrons will move from the valence band (the ground state) to the conduction band (the excited state) and can be harnessed as electrical energy.
If a photon whose energy matches the bandgap of the semiconductor hits a solar cell, this process proceeds smoothly: The incident photon generates an excited electron that is effectively captured to generate electricity. If a photon has an energy greater than the bandgap of the material—as is the case for all visible light for most photovoltaic materials in use—the incident photon generates an electron higher in energy. This excited electron then rapidly relaxes to an energy equal to the bandgap, and all the excess energy is lost as heat, a waste for the solar cell. Even worse, photons with less energy than the bandgap cannot do anything productive at all, and simply pass through the semiconductor unabsorbed.
This presents a difficult trade-off for the solar-cell designer: Wider bandgaps will lose less as heat but absorb fewer photons, while narrower bandgaps will absorb more of the available photons but lose more as heat. Silicon, the ubiquitous light-absorbing photovoltaic material that makes up more than 90 percent of today’s photovoltaic market, sits in the sweet spot of this trade-off. Still, even the best experimental silicon solar cells leave almost three-quarters of the available sunlight power unharvested.
This frustrating state of affairs has long inspired scientists and engineers, including us, to search for a better approach.
One promising idea is to use multiple absorber materials to create a stack of solar cells in which each semiconductor is paired with a particular portion of the solar spectrum. Designing these cells can be tricky. For instance, in one configuration, each subcell must output the same amount of current; otherwise, efficiency will be limited to that of the worst-performing subcell. Currently, the most efficient device made using three light absorbers under standard illumination—that is, without using lenses or other concentrators—has an efficiency of 39.5 percent.
UPCONVERTING PHOTONS
Chris Philpot
TWO LOW-ENERGY PHOTONS can be turned into a single higher-energy photon by means of triplet-triplet annihilation. First, a low-energy photon [red wave] strikes a sensitizer molecule. This boosts [blue arrow] an electron from the ground state to the singlet excited state. The excited electron in the sensitizer subsequently falls to the lower-energy triplet state and flips its spin. The sensitizer molecule then exchanges an electron for an electron in the annihilator molecule, rubrene in this case. After this electron transfer, the sensitizer’s electrons have returned to the ground state and the annihilator is in the triplet excited state. The process repeats itself, generating a second annihilator in the triplet excited state.
But we think that changing the color of light can further boost efficiencies: Instead of trying to match the cell to the incoming light, we can match the light to the cell.
That means we convert the photons below the solar cells’ bandgap to harvestable, higher-energy photons. In the last few years in our lab at Stanford and in collaboration with other scientists around the world, we have successfully upconverted low-energy infrared photons—which often can’t be used by today’s solar cells—into productive yellow photons that can. And we translated this chemistry, originally developed in a beaker, into a thin-film material.
We are studying how to improve these gains by controlling how energy moves inside our materials, how the singlet and triplet states interact, and how the light is emitted from the thin film to a solar array. Scientists around the world, including us, are working to develop materials that will enable more-efficient upconversion systems that harvest even further into the infrared. This technology isn’t being used commercially yet, but we believe it will get there.
Using Color Changes to Hit a Target
Improving the efficiency of solar cells is far from the only exciting use for changing the color of light through upconversion. This technology can also be used to target light to a precise location, solving a problem common to biology, chemistry, and additive manufacturing.
Preventing undesirable absorption or scattering of light is important in applications as diverse as activating a drug at a tumor site, lighting up a neuron to study brain function, and—perhaps surprisingly—precisely building structures through additive manufacturing. The way we solve this issue is similar in each case, but additive manufacturing (3D printing) is particularly promising and perhaps the easiest to explain.
If one were to imagine the best way to print a shape in three dimensions, without using today’s technology, it’s easy to picture curing individual points at their x, y, and z coordinates within a vat of resin. Yet curing a single target point without curing the space around it is difficult. Shining a laser beam into the resin, for instance, cures it along the entire laser path.
But we can get to this level of precision by changing the color of light. Here’s how that works.
Inside our upconversion 3D printer we use a resin containing dispersed nanoparticles with sensitizers and annihilators. In 3D printing, blue or UV photons are typically used to drive the curing of resin, but we don’t start with blue light. Instead, we shine a red laser beam toward our target.
Then we take advantage of the fact that upconversion happens only at certain intensities of light: We use a lens to focus our red beam on a particular point in the resin pool, increasing its intensity at that spot. Upconversion creates a small dot of blue light at the focal point of the red light, curing the resin at the dot. By moving the focal point around, we can create arbitrary shapes deep in our resin pool. What’s exciting is that this entire process can be run with a laser no more powerful than a typical laser pointer. We have already made a number of sample objects, including a toy boat, a gear, and some Stanford and Harvard University logos.
Moving forward, we are particularly excited about using this technique to rapidly print many objects at the nanoscale in parallel—something that has been difficult to do, since focusing too many high-powered lasers into one pool of resin simply breaks the resin down before it can be transformed into a solid plastic. The low-power lasers used for upconversion don’t do this.
Still further promising developments remain beyond these applications: Upconversion could allow for near-infrared beams, which penetrate deep into living tissue, to generate high-energy photons useful for deep-tissue imaging, optogenetics, and local chemical reactions.
Finally, we’re also exploring applications like passive night-vision systems and robust anticounterfeiting schemes. Each of these applications would require a thin coating of upconversion materials on a surface, in the same way we’re using our technology with solar cells. Imagine purchasing a pair of glasses with an added upconversion coating that allows you to see infrared photons, improving night vision without the bulky power source required in today’s night-vision goggles. Or, if you embedded upconversion nanoparticles in currency or ID cards, distinguishing real from counterfeit would be as easy as shining a red laser pointer at the surface and seeing the light turn blue.
Although each application requires different types of materials, getting high-energy photons to the right place through upconversion can be used to kick-start each one. We’re only beginning to scratch the surface of what this technology can do.


