On 21 March, an advisory panel to the U.S. Food and Drug Administration gave its unanimous blessing to a piece of wearable technology that alleviates symptoms of the progressive eye disease glaucoma.
Known as the FSYX Ocular Pressure Adjusting Pump (OPAP) system, the device is designed to ease the pressure that accumulates in the eyes of people with glaucoma. This elevated pressure poses a risk of damaging the optic nerve, which relays visual information to the brain, leading to irreversible vision loss.
“This will give our toughest-to-treat patients additional options,” says John Berdahl, an ophthalmologist in Sioux Falls, S.D., and the founder of Balance Ophthalmics, the company developing the OPAP system.
Full regulatory approval is expected in the coming months. While the FDA is not required to adhere to its panels’ recommendations, it typically does so. According to Berdahl, Balance then plans to seek approval in other parts of the world. East Asia, a region with high incidences of the type of glaucoma addressed by the device, is a top priority, he says.
Pressure Point
Glaucoma remains a leading cause of blindness globally. Current treatment strategies include eye drops, laser treatments, and surgical procedures—but each has its limitations.
The OPAP system is designed for nighttime use, equipped with a programmable vacuum pump that delivers negative pressure to the eyes via a pair of snugly fitting goggles.
Eye drops can cause irritation, and they require diligent adherence. Surgery comes with the potential for infection, scarring, and other complications. And laser treatments cannot adequately reduce eye pressure in severe glaucoma cases.
What’s more, none of these interventions provide a quick fix to the ongoing challenge of maintaining proper pressure within the eyeball—what clinicians refer to as intraocular pressure (IOP).
“The biggest void that we have currently in glaucoma care is the ability to instantaneously modify a patient’s IOP,” says Brian Shafer, an ophthalmologist in Plymouth Meeting, Pa.
The wearable OPAP device thus offers a much-needed alternative, one that can be dialed in to the specific pressure needs of each glaucoma patient.
With this setup, “you’ve got this super-modifiable, titratable, instantaneous method to adjust IOP—and we don’t have that with anything else,” says Shafer, who consulted for a now-defunct precursor company to Balance.
If it receives FDA go-ahead, OPAP would become the first nonsurgical, nonpharmacological, purely physics-based treatment option for glaucoma—in particular, for a form of the disease known as “normal-tension glaucoma” in which IOP is not raised outside of typical levels yet the pressure wreaks havoc on the optic nerve nonetheless.
Transforming the Negative
According to Balance CEO Seph Jensen, the company chose the name OPAP to mirror the functionality of CPAP machines, which assist individuals with obstructive sleep apnea.
In line with those sleep-apnea devices, the OPAP system is designed for nighttime use and is equipped with a programmable vacuum pump that delivers negative pressure to the eyes via a pair of snugly fitting goggles.
This negative pressure is thought to modify fluid dynamics within the eye and its supporting blood vessels. Those changes in turn help to limit mechanical strain on the optic nerve head, the funnel through which more than a million nerve fibers from the retina come together to transmit visual signals to the brain.
“And basically,” says Ross Ethier, a bioengineer at Georgia Tech who consults for Balance and has modeled these effects, “everything we know about glaucoma tells us that that will be beneficial for patients if they wear the device.”
Published experiments with cadavers and brain-dead organ donors—individuals who have consented to allowing such research studies—largely bear this out. (The cadaver study, it should be noted, was funded by Balance’s predecessor company, although the organ-donor study was funded by foundation and government grants.)
In cadavers, for example, Shafer found that the negative pressure exerted by the OPAP system altered pressure within the eye, but did not change pressures further back in areas leading toward the brain. This normalization of the pressure gradient across the back of eye is thought to help stop the damage that can lead to glaucoma.
“It proved that the theory behind it actually works,” Shafer says.
Moreover, in a yearlong clinical trial, around 90 percent of people who wore the goggles nightly experienced a dramatic drop in IOP in their treated eyes, with no major safety issues. (The most common side effect was mild swelling in the eyelids or surrounding tissue.) By comparison, less than 5 percent of control eyes achieved this same pressure reduction on their own.
The 93-person trial was not designed to test the device’s ability to forestall visual impairment—the primary concern for individuals with glaucoma. However, says Jeffrey Goldberg, an ophthalmologist at Stanford University who led an earlier pilot study, “reducing nighttime eye pressure is highly likely to protect patients with glaucoma from vision loss over time.”
Berdahl and his clinical collaborators presented the unpublished trial data at the 21 March FDA online advisory committee meeting.
Goggle Glitches
As it currently stands, the device does not meet the FDA’s latest cybersecurity standards. That means users will have to physically connect their goggles via USB cable to upload data in a clinician’s office.
Data tracking is essential for patient compliance, insurance reimbursement, and aiding in disease management, says the company. In the future, Balance hopes to release a Bluetooth-connected version that can securely transmit user information to the cloud.
The OPAP system is more like a CPAP machine than its inventors necessarily anticipated. Both seem to be highly effective—but only if worn correctly and consistently.
Another limitation: the goggles can make people uncomfortable as they sleep. Just ask Joseph Kim.
“I couldn’t handle the therapy,” he says.
Kim has long worked in the clinical-trial arena, helping to optimize the experience for patients and to streamline protocol tasks for study investigators. So when, shortly after being diagnosed with glaucoma, Kim was invited to participate in a trial for the OPAP system, he went for it. “I jumped at the chance,” he says.
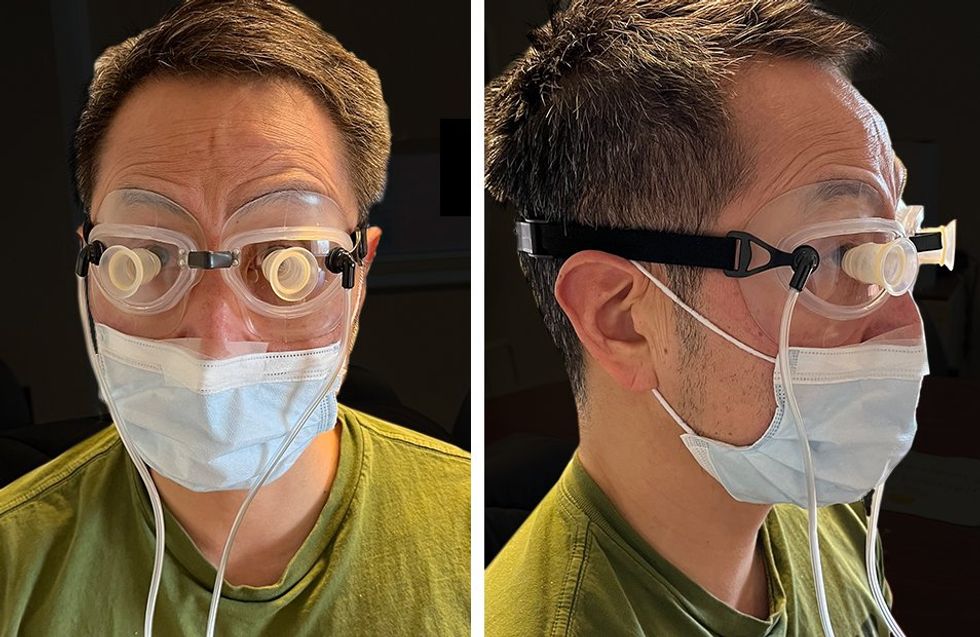
Kim says he didn’t mind applying eye drops each night. But the OPAP trial promised more than just treatment; it offered a window into the patient’s journey through the eyes of a participant.
“It seemed like a great way to get more firsthand knowledge about the patient experience in an honest way,” says Kim, chief strategy officer for ProofPilot, a company that develops software to support medical trials.
He says he struggled with the device, though. Kim normally sleeps on his side, but doing so often jostled his goggles, breaking the pressure seal, and triggering alarms that would wake him up. Sweat would also build up under the goggles, causing irritation and unease.
Faced with consecutive nights of poor sleep, Kim—like around one-third of all participants who started with the OPAP system in its pivotal clinical test—made the decision to withdraw from the trial.
“Listen, I’m in the business of clinical research,” he says, “and I just couldn’t do it—sadly.”
In this way, the OPAP system is more like a CPAP machine than its inventors necessarily anticipated. Both seem to be highly effective—but only if worn correctly and consistently. And many would-be beneficiaries could have trouble with device tolerance.
Therefore, says Toh Song Tar, a sleep-apnea specialist at Singapore General Hospital who has studied the connections between apnea and glaucoma, “patient counseling will be important.”
“If people understand that the OPAP device will help to protect their eyesight against the ill effects of glaucoma, it will help and they will be more diligent in using it,” he says.
The success of the OPAP system, potentially the first wearable technology for glaucoma care, could therefore depend less on the technical aspects of applying negative pressure to the eye, which has been the focus of Berdahl’s research for the past decade.
Instead, it could hinge on an unpredictable factor: human behavior and the willingness of glaucoma patients to stick with the technology.
- Teen Wins Scholarship for His Glaucoma-Detection Device ›
- New AI Test Diagnoses Glaucoma in Just 10 Seconds ›
Elie Dolgin is a science writer specializing in biomedical research and drug discovery. After a PhD spent studying the population genetics of nematodes, he swapped worms for words—entering journalism as an editor at The Scientist, Nature Medicine, and STAT. Now a freelancer, Elie is a frequent contributor to New Scientist, Nature, IEEE Spectrum, and more.



