Glaucoma affects 65 million people and is the second-most common cause of blindness in the world. One of its main risk factors is an increase in the eyeball fluid pressure, which can build up enough to damage the optic nerve. Eye doctors today measure this intraocular pressure using a tonometer, but the test is not always accurate.
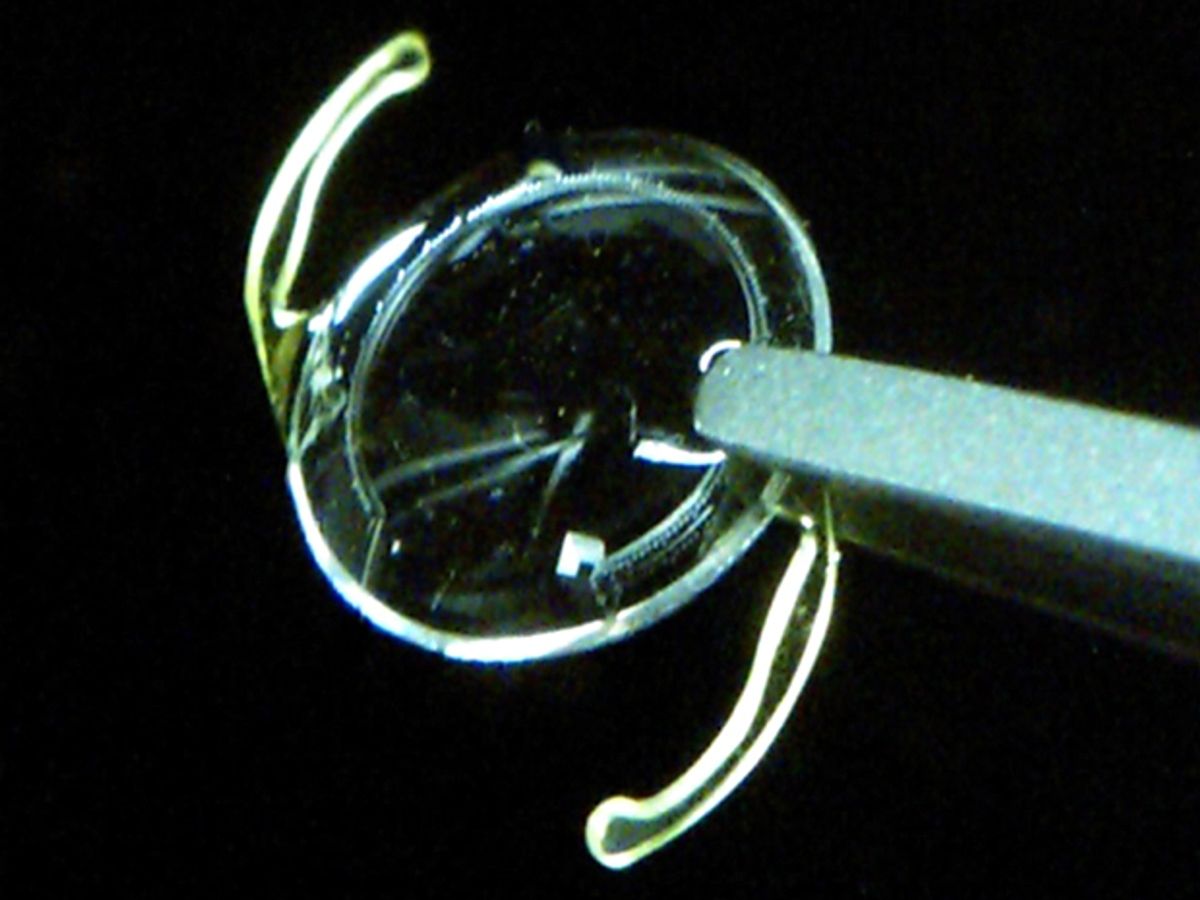
The new sensor consists of an airtight 50 µm-channel that runs around most of the periphery of a lens that is used for cataract surgery. On one side it ends in a tiny gas reservoir, while on the other it connects to the aqueous eyeball fluid. A doctor would surgically implant the lens into a patient’s eye.
When the microchannel is connected into the eye chamber, pressure drives the intraocular fluid into the microchannel, compressing the reservoir gas until the gas pressure and liquid pressure reach equilibrium. An increase or decrease in the intraocular pressure forces the fluid to move toward or away from the gas reservoir. A smartphone camera equipped with an optical adapter and image analysis software can be used to accurately detect the position of the liquid. The optical adapter positions the camera in front of the pupil and shades the eye, causing the pupil to dilate and reveal the sensor.
Yossi Mandel of Bar Ilan University in RamatGan, Israel and Stephen Quake of Stanford University and their colleagues reported the new sensor in the journal Nature Medicine.
The researchers first tested and calibrated the sensor in a pressure chamber by simulating changes in intraocular pressure. They found that the movement of the liquid inside the microchannel was linear to pressure changes and sensitive to pressure fluctuations as small as 1 mm Hg. Normal intraocular pressure ranges between 10-21 mm Hg, but can increase by 8 mm Hg when a person is lying down. The researchers also tested the implant in surgically removed pig eyes, where it also showed a detection limit of 1 mm Hg.
Other eye pressure sensors exist. University of Michigan researchers have developed, for instance, microelectromechanical system-based capacitive sensors. And Swiss medical device-maker Sensimed already has a commercial contact lens-based eye pressure sensor in which a piezoelectric platinum ring changes resistance when the eyeball inflates. But these approaches rely on wireless data telemetry, which requires bulky antenna and power sources.
The optical readout on the new microfluidic sensor could be easier to use, though it does have its own limitations. Reading the fluid position through a hazy cornea, which can happen in glaucoma patients, could be difficult, for instance. And gas could leak out of the sensor walls, making readings inaccurate. Nevertheless, the researchers say that their experimental results suggest a 10-year device life.
Prachi Patel is a freelance journalist based in Pittsburgh. She writes about energy, biotechnology, materials science, nanotechnology, and computing.