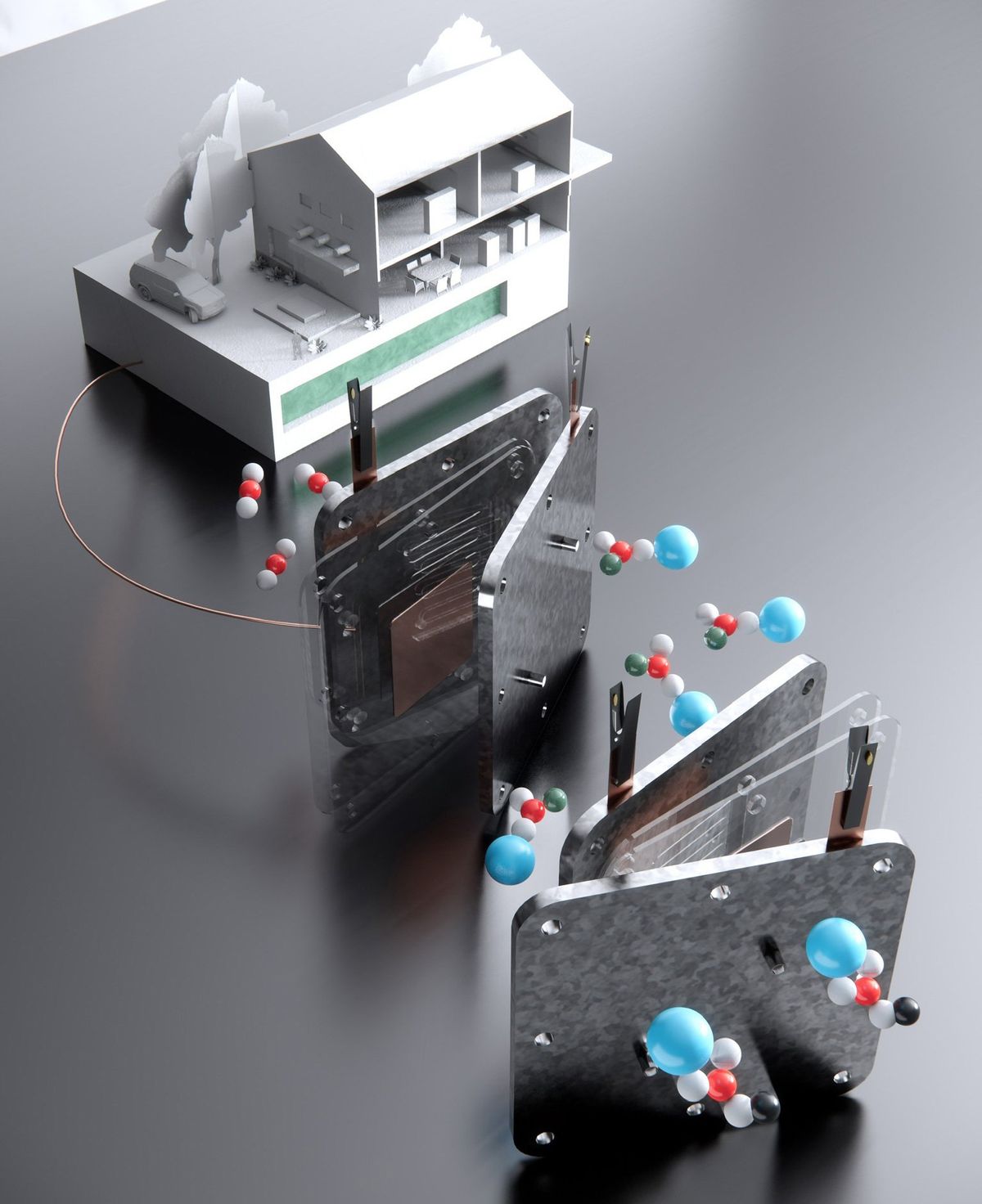
Dozens of eager research efforts are underway around the world to capture carbon dioxide from power plants, the air, and even the oceans. Although that greenhouse gas can be stored underground, it would be great if it could be turned into fuel.
Researchers at MIT and Harvard University have now reported an electrochemical process that turn carbon dioxide into formate, which, as a liquid or solid, can be used as in fuel cells to produce electricity. The process is 95 percent efficient, way ahead of the 10 to 20 percent efficiencies of the other known carbon dioxide-to-fuel methods.
“It already takes a lot of energy to capture carbon dioxide, so when we utilize it to make chemicals or fuel, we need high efficiency,” says Ju Li, a professor of nuclear science and engineering and materials science and engineering at MIT. “Second is that the product needs to be useful. Our goal was to find a stable form of fuel that can be seasonal. You could make it in the summer when electricity is abundant, to be used in the winter or even on a multiyear time scale.”
Li and colleagues reported their findings in Cell Reports Physical Science.
Several teams have been working on mimicking the photosynthetic prowess of plants to convert carbon dioxide to fuel. Researchers have succeeded in making artificial leaf devices that soak sunlight and carbon dioxide from air to produce hydrogen or syngas fuels. But the efficiencies of such devices typically is typically under 10 percent. Another common approach to make fuels from carbon dioxide involves two steps. The gas is first captured and turned into a solid carbonate, and then the carbonate is heated to release the carbon dioxide for conversion into a fuel.
Hydrogen fuel is also hazardous and requires storage in high-pressure tanks, one reason the hydrogen economy has been slow to take off. “The flash point is very low, which can cause explosions,” says Li. “Second, it’s not a very good seasonal fuel because we lose 1 percent of hydrogen per day because the gas gets into metals, and it leaks.” Some groups are also working on converting carbon dioxide into energy-dense methanol fuel, which is toxic, says Li.
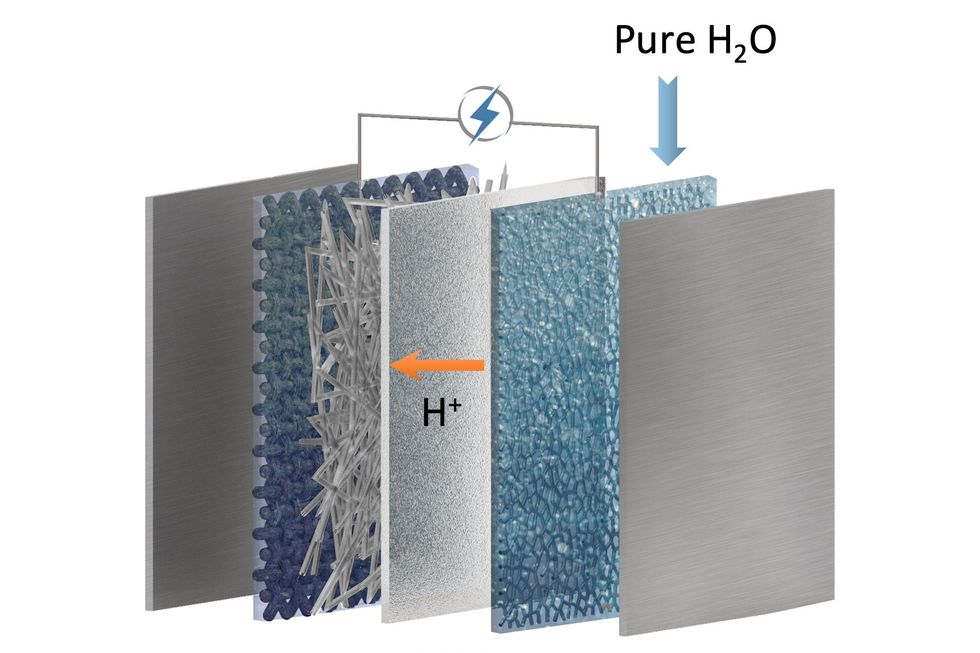
The researchers start by putting carbon dioxide in an alkaline solution to make liquid potassium or sodium bicarbonate solutions. Next, they run the solutions through an electrolyzer, where an electric current converts the liquid bicarbonate to liquid formate. “What we do here is a liquid-to-liquid conversion, which gives the high efficiency.”
Then the researchers evaporate the liquid under sunlight, leaving solid formate crystals, which are easy to store. When power is needed, the solid powder would be mixed with water and fed into a fuel cell, Li says.
For now, the researchers built a palm-size electrolyzer device in the laboratory, which they could run for more than 200 hours with no decrease in formate output. They also made a direct formate fuel cell (DFFC) that runs on metal formate to produce electricity. Once scaled up, the fuel cell will have an energy density of 2.13 kilowatt-hours per kilogram, more than five times that of state-of-the-art lithium-ion batteries, the team reports. It had a peak power density of 302 milliwatt per square centimeter, which they write “is already quite competitive” against the standard hydrogen proton-exchange membrane fuel cell, which has 700 mW/cm2.
Li and colleagues propose to get bicarbonate from natural sources. Certain mineral rocks found around the world are known to capture carbon dioxide from the atmosphere. Li says that the insoluble components of such rocks could be separated and buried for carbon sequestration, while the soluble potassium and sodium bicarbonates could be converted in their electrolyzer to metal-formate fuels.
“Thus, the combination of high-efficiency bicarbonate-to-formate electrolyzer and DFFC should enable a ‘solid formate economy,’ ” the team writes.
- Carbon Storage and Hydrogen: Match Made in Heaven? ›
- Future Trains Could Provide Carbon Capture on Wheels ›
- Fuel Cells Finally Find a Killer App: Carbon Capture ›
Prachi Patel is a freelance journalist based in Pittsburgh. She writes about energy, biotechnology, materials science, nanotechnology, and computing.