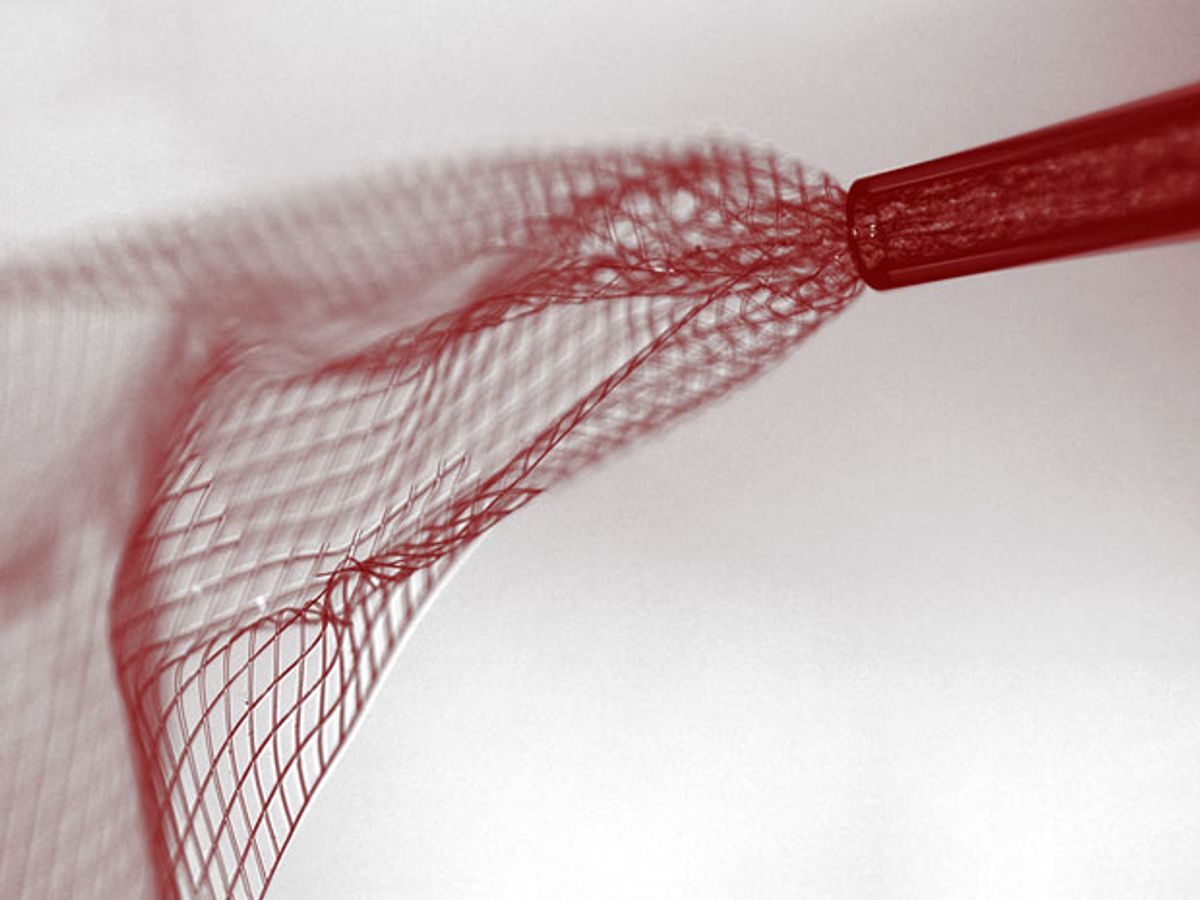
Tiny electronic meshes that can be injected directly into the brain might one day provide control of prosthetic limbs, repair brain damage, or make artificial eyes, according to scientists who have already inserted the devices into the brains of mice.
The team created a mesh of simple field-effect transistors out of silicon nanowires, then put them in a saline solution, drew them up into syringes, and injected them into the mouse brains. The meshes rolled up like tiny scrolls when they were drawn into the needle, then relaxed back to their natural shape over the course of about an hour once they’d been injected.
The researchers, who have been working on nanowire electronics for several years, noticed that their nanowire mesh naturally curled up when placed in liquid. “It looked like a polymer, and we thought, ‘now maybe we could just suck this stuff into a syringe and inject it,’” says Charles Lieber, a chemistry professor at Harvard whose group reported their research online in Nature Nanotechnology this week. “It’s a pretty common way of delivering things in biology and medicine.”
Back in 2012, Lieber’s team built silicon nanowire scaffolds and showed they could grow nerve, heart, and muscle tissue on them with the aim of sensing how the cells respond to stimuli. The new meshes, also made of silicon, are much flatter than the scaffolds, and are shaped like parallelograms, which helps them to curl up to a smaller diameter than that of the needle. When unfurled, the meshes were a couple of centimeters wide—33 times the diameter of the needle—and 3 or 4 cm long. Conductive polymer leads attached to the meshes extended to the outside of the mouse’s head, providing input/output access to the electronics.

Flexibility is important for interfacing with the brain, and the meshes are about 100,000 to 1 million times as bendable as conventional flexible electronics. “We want it really soft. We don’t want it cutting and damaging the tissue,” Lieber says. Stiffer devices, such as the electrodes implanted in Parkinson’s patients for deep brain stimulation, cause scarring, which insulates the electrodes, so doctors have to reposition them and turn up the charge over time, leading to unpleasant side effects. The individual features on the mesh are smaller than brain cells, which also seems to reduce the odds of an immune reaction.
In the mice they tested, the signal actually improved over three months. And the team saw cells migrating hundreds of micrometers across the mesh in a month’s time. That suggests the meshes integrate well with brain cells, and might even be useful as patches for damage caused by a stroke or spinal cord injury. “You’re almost making sort of artificial synapses with this,” Lieber says.
It will be several years before this technology can be used in humans; the team is still trying to determine if the meshes remain stable for as long as three to six months. The fact that there are U.S. Food and Drug Administration approved implants such as deep brain stimulation, however, could provide an entree for testing the meshes. Further down the road, Lieber imagines injecting meshes along with stem cells, or altering their surface chemistry to bind them to particular types of cells, or perhaps building more complex electronics with more capabilities. “There are many, many things you can think about,” he says.
Neil Savage is a freelance science and technology writer based in Lowell, Mass., and a frequent contributor to IEEE Spectrum. His topics of interest include photonics, physics, computing, materials science, and semiconductors. His most recent article, “Tiny Satellites Could Distribute Quantum Keys,” describes an experiment in which cryptographic keys were distributed from satellites released from the International Space Station. He serves on the steering committee of New England Science Writers.