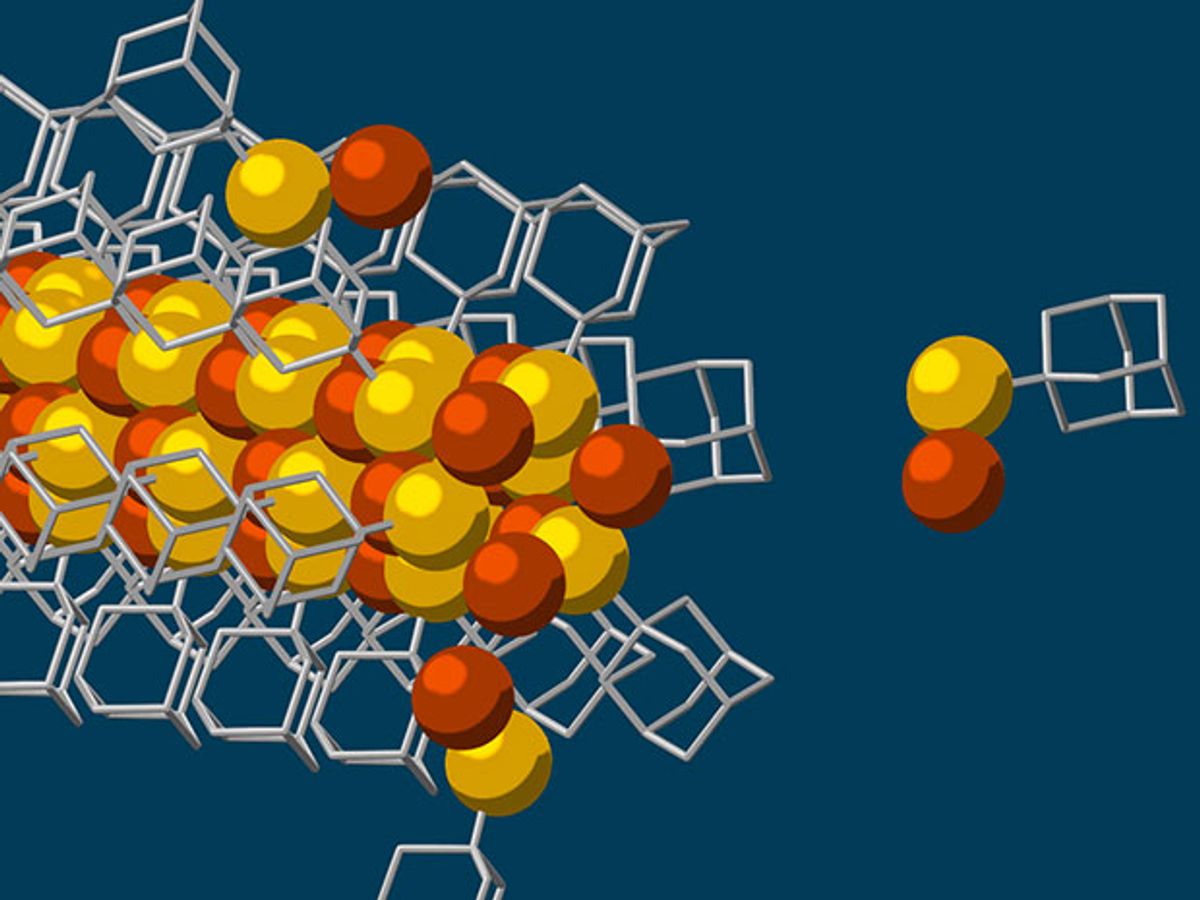
A team of researchers from Stanford University and the US Department of Energy’s SLAC National Accelerator Laboratory have developed a self-assembly process that uses diamondoids to create nanowires with a solid, 3-atom wide copper-sulfur crystalline core—the smallest possible.
The resulting nanowires possess superior electrical properties due to the lack of defects present in the solid crystalline core. Perhaps more impotantly, the self-assembly process for making the nanowires could lead to new kinds of optoelectronic devices and superconducting materials.
“Achieving a 'solid core' of a three atom cross section is ideal,” says Nicholas Melosh, an associate professor at SLAC and Stanford, in an e-mail interview with IEEE Spectrum. “It’s small enough to exhibit unique functionality, yet it can tolerate single defects or strains since there is still a pathway for the electrons to flow.”
In research described in the journal Nature Materials, the SLAC team took a different approach to traditional self-assembly techniques for growing nanowires, and it was this difference that resulted in the remarkably better electron conductivity.
This self-assembly process starts with diamondoids, which are the smallest form of diamonds and in this case are just tiny interlocking cages of carbon and hydrogen atoms. These diamondoids are attracted to each other through van der Waals forces, attractive or repulsive forces that exist between molecules or groups of atoms.
In a process that is essentially a mixing pot in which all the ingredients are added into a single pot, the scientists added sulfur so that a sulfur atom attaches itself to each of the diamondoids. While floating in this solution, the sulfur atom bonds with a single copper ion and this formed the basic building block for creating the nanowires. While floating in this solution, the van der Waal forces working on the diamondoids drew these building blocks together to grow the nanowire.
“Other molecular self-assembly methods have been tried, yet balancing the delicate interplay between attractive and repulsive forces to get just the size you want has proven very difficult,” said Melosh. The diamondoid molecules that Melosh and his colleagues used for this study provided a unique combination of strong intermolecular attractions along with a group of atoms that take up just the right amount of space, according to Melosh.
Melosh believes that these nanowires (and the synthetic process to make new materials) open up a lot of possibilities to move some of the exotic physics found in ultra-thin materials to the world of the macroscopic. Topological insulators are one example Melosh gave. These are materials that behave like conductors near their surfaces but act as insulators throughout the bulk of their interiors.
“Bulk versions of these materials do not show these new properties, requiring nanoscale versions of the materials, which are generally difficult to make and to handle,” said Melosh. “Our approach is to make the materials inherently have these properties. In our materials, every single nanowire has these unusual properties, and the bulk material does too, since its composed of billions of individual nanowires. This should also hold as we go to meshes or fabrics, which are not possible with current materials.”
There are more conventional materials that these nanowires could find their way into, like piezoelectrics in which motion can be converted into electricity.
“You can imagine weaving those into fabrics to generate energy,” Melosh said in a press release. “This method gives us a versatile toolkit where we can tinker with a number of ingredients and experimental conditions to create new materials with finely tuned electronic properties and interesting physics.”
In continuing research, Melosh and his colleagues are exploring new atomic elements to discover what materials are even possible and what properties they have, then extending the processing to create bulk materials from the nanowires.
Dexter Johnson is a contributing editor at IEEE Spectrum, with a focus on nanotechnology.