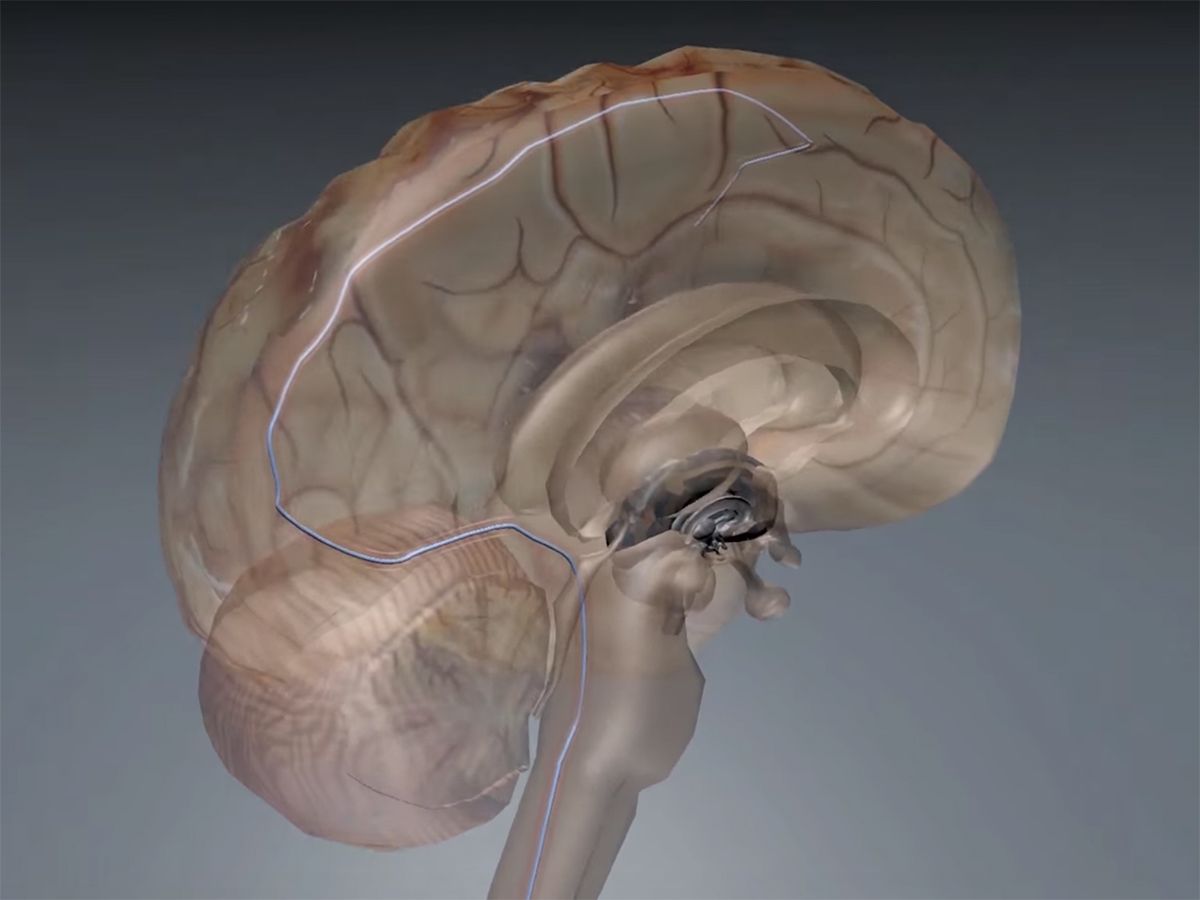
In 2016, Australian scientists announced a matchstick-size brain implant that can be slipped underneath the skull via blood vessels—similar to how a pacemaker’s leads are eased into the heart. The stent electrode, or “stentrode,” recorded high-quality brain signals in freely moving sheep for six months.
Now, the stentrode adds another ability to its arsenal: In addition to monitoring brain signals, the device can communicate with the brain using gentle pulses of electricity.
In a proof-of-concept study published this week in Nature Biomedical Engineering, the team used stentrodes to electrically stimulate the motor cortex of sheep brains, eliciting movement in the animals’ facial muscles and limbs.
That ability suggests that the device could be used to perform deep brain stimulation (DBS) in humans, a form of direct electrical simulation shown to be a promising treatment for conditions such as Parkinson’s disease, depression, and epilepsy.
Implanting traditional DBS electrodes requires drilling a hole through the skull or removing a portion of it. The stentrode, on the other hand, is implanted into the brain by snaking a catheter underneath the skull via a vein in the neck. Pacemakers are similarly implanted in the heart in a procedure that takes about an hour and requires only local anesthetic—the patient is typically awake the whole time.
“Our technology potentially is an avenue to achieve deep brain stimulation without performing open brain surgery,” says Thomas Oxley, CEO and founder of Synchron, the Silicon Valley–based company developing the technology.
That could make DBS a more accessible and less-expensive treatment option for patients. And unlike some brain implants, the stentrode has caused no brain inflammation or rejection in studies so far.
In a side-by-side comparison in sheep, the stentrode stimulated brain tissue as well as a traditional implant. During the study, the team also discovered that the direction in which the electrode is facing inside the blood vessel can affect how much energy is required to stimulate the brain—an important piece of information to consider as the trials move into human studies.
In 2016, Oxley predicted the first human trials would begin in late 2017. That didn’t happen, and he now declines to put a date on the start of clinical trials. Like a pacemaker, the stentrode implant is permanent, which makes human trials a significant undertaking.
“The burden is on us to get the technology to a position where it’s really safe when we do that first [human] implant,” says Oxley. “We’re $17 million and six years into this program and only now getting really close to our first in-human trial.”
That first human trial, focused on safety, will enroll patients with paralysis, says Oxley. The company’s initial goal is to develop a brain-computer interface that would allow individuals with paralysis to mentally control devices such as wheelchairs, prosthetic limbs, or computers.
Eventually, the ability to stimulate the brain will be a valuable addition to a brain-computer interface, he adds. “An ideal brain-computer interface would contain a closed loop with a feedback circuit, so we could very quickly provide information back to the brain.”
Megan is an award-winning freelance journalist based in Boston, Massachusetts, specializing in the life sciences and biotechnology. She was previously a health columnist for the Boston Globe and has contributed to Newsweek, Scientific American, and Nature, among others. She is the co-author of a college biology textbook, “Biology Now,” published by W.W. Norton. Megan received an M.S. from the Graduate Program in Science Writing at the Massachusetts Institute of Technology, a B.A. at Boston College, and worked as an educator at the Museum of Science, Boston.