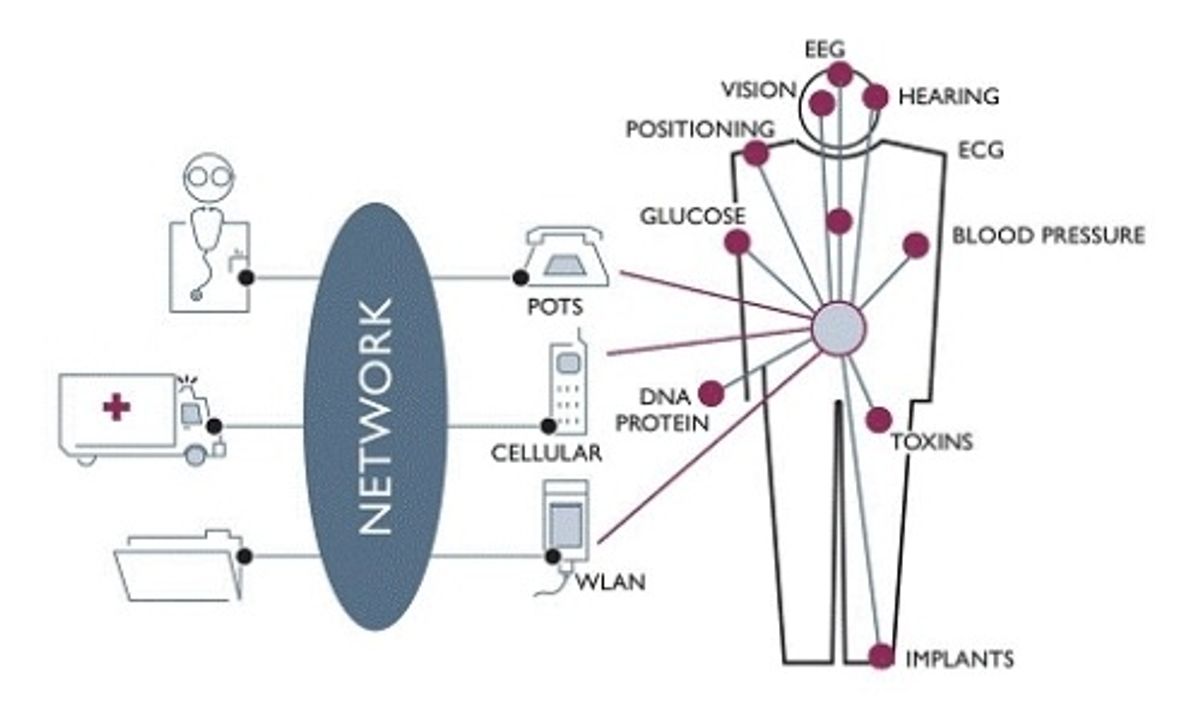
Electronic health monitoring took a big step forward last week when the U.S. Federal Communications Commission approved spectrum for medical body area networks (MBANs). The FCC allotted the frequencies between 2360 and 2400 megahertz, which were part of the spectrum that was returned to the commission when TV shifted from analog to digital.
An MBAN is a network of inexpensive disposable sensors worn on, or even implanted in, the body. The sensors monitor various vital signs, such as temperature, blood pressure or glucose levels, and transmit the information to a control device. The technology holds the promise of getting rid of the myriad wires that tie patients to their hospital beds today—wires that complicate patient care and pose infection risks. The first use of MBANs will likely be in hospitals, but a longer term application could be remote monitoring devices that allow doctors to check on patients at home.
FCC Chairman Julius Genachowski said the decision opened up “tremendous potential to untether patients from tubes and wires, and improve the quality of health care and ensure better outcomes for patients.” He cited estimates predicting that MBAN monitoring could save an average of U.S. $12,000 per patient by decreasing hospital-acquired infections.
Among the other benefits of MBANs:
- The networks can potentially alert doctors to problems before the patient’s condition becomes critical, thus avoiding the need for acute intervention.
- The lack of multiple wires makes it easier for hospital staff to move the patient, and gives the patient more freedom to walk around.
- Hospital personnel or home caregivers can quickly add or remove sensors as medical conditions warrant.
Several FCC commissioners hailed the decision as a blueprint for how industries can cooperate in sharing spectrum, alluding to ongoing skirmishes between the defense, broadcasting, and technology industries about how to use limited spectrum resources. GE Healthcare had first proposed allocating specific spectrum to MBANs in 2007, but was met with opposition from the aerospace industry, which uses part of that spectrum for flight testing. It took five years, but the two industries reached a compromise. “This proceeding affirms what is possible when members of our communications industry work past initial disagreements,” said Commissioner Mignon L. Clyburn. “Perhaps the details of their approach can be followed to promote sharing in other bands as well.”
Healthcare device makers and the aerospace industry “have served as a model for developing shared use policies for spectrum that address interference concerns while allowing new services to flourish,” said Commissioner Jessica Rosenworcel. “With the growing demand for spectrum resources, it is cooperative efforts like this that give us hope and faith.”
Although MBANs have cleared a major hurdle, it will be at least another year before they hit the market. First, there is a public comment period, and then the FCC must set up rules for registration and frequency coordination. The Commission will also have to approve and certify MBAN products, just as it does for all radio-frequency equipment. And because MBAN equipment will be sold as medical devices, they will also have to gain approval from the U.S. Food and Drug Administration.
Freelance journalist Tam Harbert has covered technology and business for more than 20 years. Based in Washington, D.C., Harbert says her favorite type of article explains how public policy affects the technology business, or vice versa. She has launched, edited, and written for publications targeting engineers, IT managers, investors, and corporate executives. Harbert has won more than a dozen awards for her work, most recently two awards (2014) from the American Society of Business Publication Editors (ASBPE) and the American Society of Journalists and Authors (ASJA). She has also received the Jesse H. Neal Award for op-ed writing.