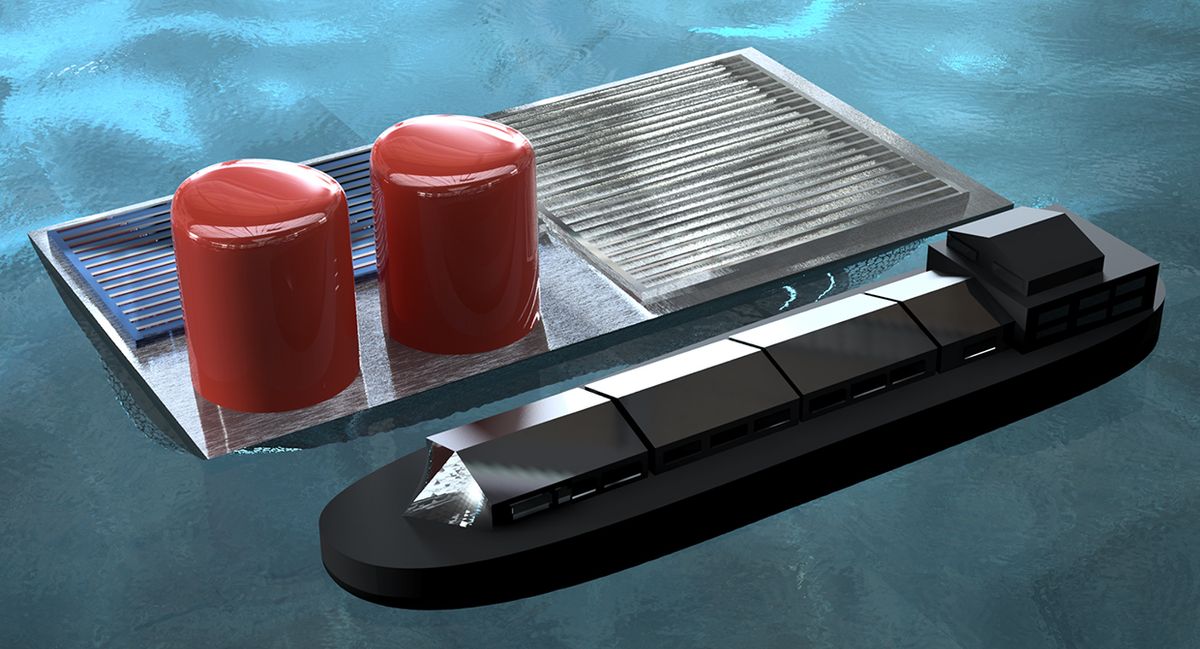
A floating “solar fuel rig” could one day use solar energy to split apart seawater and generate hydrogen fuel. A team of scientists recently described the design for the new rig in the International Journal of Hydrogen Energy. A scaled-up version of their prototype could someday float out on the open sea, they say, producing renewable fuel from sunlight and seawater.
Scientists have long sought ways to harness sunlight to produce storable fuels that could be put to work when the sun doesn’t shine. One strategy aims to use solar-generated electricity to drive the electrolysis of water—the splitting of water (H2O) into hydrogen (H2) and oxygen (O2). Hydrogen is a clean fuel, the burning of which generates only water as its byproduct.
When commercial electrolyzers split water, they rely on membranes to separate the resulting hydrogen and oxygen gases, since mixtures of these gases are potentially explosive. But these membranes are expensive, and prone to degradation and failure.
Moreover, solar-powered hydrogen fuel production would ideally make use of cheap, abundant seawater. But commercial electrolyzers require very pure water, because seawater contains impurities and microbes that can easily destroy their membranes.
Previously, scientists had tried to develop electrolyzers that did not require membranes. However, these electrolyzers used flowing electrolyte fluids to separate hydrogen and oxygen, which required pumps and added a layer of mechanical complexity to these devices.

Now researchers have developed what they suggest is “the first demonstration of a practical, floating photovoltaic-driven electrolysis device that is membrane-free and pump-free,” says Daniel Esposito, a chemical engineer at Columbia University, in New York, who helped build the rig.
Esposito and his colleagues envision platforms of their solar-powered electrolyzers floating on the sea to make use of abundant sunlight and water to generate hydrogen fuel. These “solar fuel rigs” are reminiscent in some respects of the deep-sea rigs used to harvest fossil fuels today, they say. “About 71 percent of the Earth’s surface is covered by water—why not use some of that space to harvest energy?” Esposito says.
The new electrolyzer uses electrodes made of sheets of titanium mesh that are suspended in water. A platinum catalyst coats just one side of each sheet. “These mesh electrodes are similar in nature to the metal window screens that allow desirable exchange of air between indoors and the outdoors while keeping bugs out,” Esposito says.
When a mesh electrode is negatively charged, hydrogen bubbles develop on the side coated with the catalyst. When a mesh electrode is positively charged, oxygen bubbles develop on the catalyst-coated side instead.
The mesh electrodes are each placed at diagonal angles in the water. When the bubbles of gas on them grow large enough, the bubbles’ buoyancy makes them detach from the mesh and float upward unimpeded. Depending on the arrangement of these electrodes, hydrogen bubbles can float into one set of chambers while oxygen bubbles can float into a separate array that vents the oxygen out into the atmosphere. The simplicity of the device suggests that relatively few parts are required, which lowers the material and assembly costs, Esposito says.
In experiments, the floating electrolyzer the researchers developed could produce hydrogen gas with a purity as high as 99 percent. “By using these electrodes, we can achieve efficient electrolysis with high product purity without a membrane and without a pump,” Esposito says.
Future work can refine this design for more efficient operation in real seawater. For example, the researchers suggest developing catalysts that do not generate toxic chlorine gas from chloride ions dissolved in seawater. The researchers also plan to develop modular versions of their device that are suitable for implementation in large-scale rigs.
Charles Q. Choi is a science reporter who contributes regularly to IEEE Spectrum. He has written for Scientific American, The New York Times, Wired, and Science, among others.