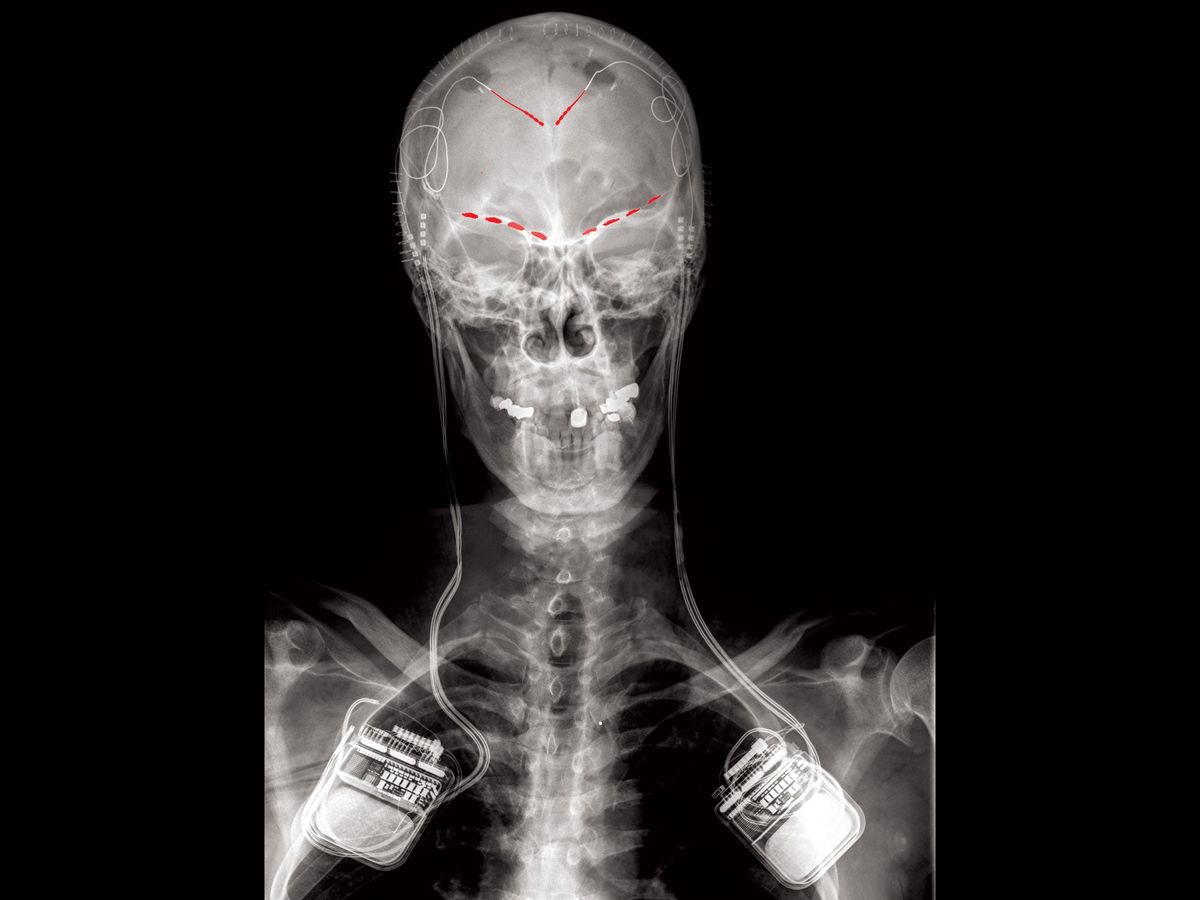
Scientists and clinicians at the University of California, San Francisco, are developing new methods of studying—and potentially treating—chronic pain. In a recent paper published in Nature Neuroscience, Dr. Prasad Shirvalkar and his team of doctors and researchers studied how types of chronic pain are represented in recordings of brain activity.
Shirvalkar, a neurologist and pain specialist with the UCSF Pain Management Center, is researching and treating central pain syndrome, a type of chronic pain produced by dysfunction within the central nervous system. Treating this condition is difficult, partially because it isn’t produced from an external source or damaged body part. Further complicating the matter is a lack of concrete knowledge about how different types of pain, both acute and chronic, are represented in the nervous system. “Things don’t happen in brain regions. They happen in distributed circuits of coordinated cells,” says Shirvalkar, referring to how pain is represented in patterns of neural activity. “Certain brain regions might contribute certain types of information processing, but by themselves they don’t accomplish any particular task.”
One treatment for chronic, neuropathic pain like central pain syndrome is to use deep brain stimulation (DBS) to manipulate the neural activity that’s causing painful sensations. DBS is also used to treat other neurological conditions like Parkinson’s and more severe cases of depression. In chronic pain cases, however, it is difficult to know exactly where to place DBS electrodes such that stimulating them will effectively shunt the brain activity causing pain.
To get a better sense of how central pain emerges from brain function, the present study looked at neural activity recorded from four patients with central pain syndrome—three experiencing chronic pain while recovering from a stroke and one experiencing phantom limb pain following an above-the-knee amputation of the right leg—and related the neural activity to questionnaires and 0 to 10 pain scores reported by each patient. The researchers recorded data from intracranial electrodes placed in the patients’ orbitofrontal cortices (OFC) and anterior cingulate cortices (ACC), two brain regions previously associated with pain sensations.
Each patient underwent surgery to have a neural recording and stimulation platform implanted into the brain. This device was portable, allowing patients to go about their lives and to record data that represented their everyday experiences with central pain. Patients were periodically reminded by a phone app to note their current pain level and record 30 seconds of brain-wave data (electrocorticograms and local field potentials) from the neural implant.
Shirvalkar and his collaborators used machine learning to relate features of neural activity to the pain levels each patient reported just prior to each brain recording. These features, which represent the relative presence of different frequencies in neural recordings collected from separate locations in the ACC and OFC, were found to reliably correlate to high and low pain states within each subject. However, the patterns of these associations—what frequencies had the most power in which brain locations—varied considerably from one patient to the next.
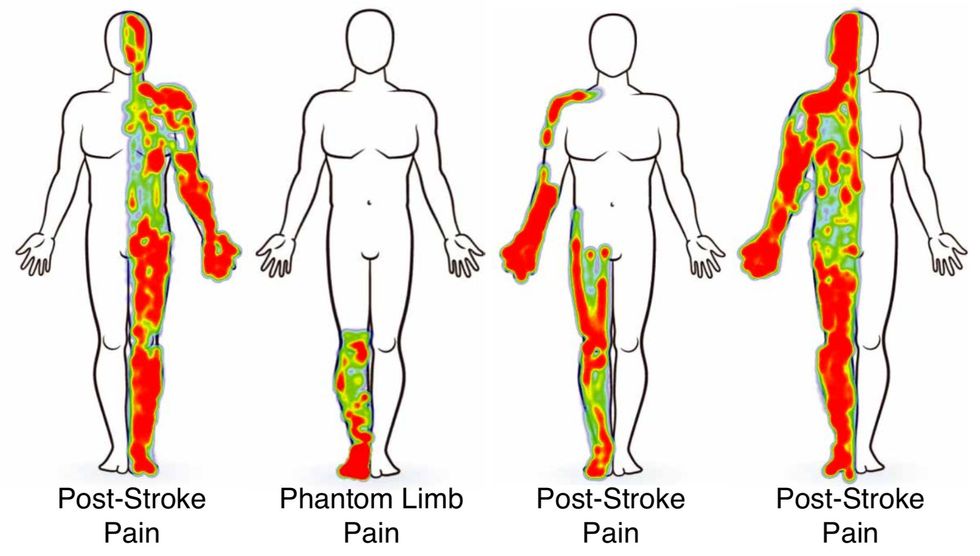
Despite the differences they observed in chronic pain-related brain activity between patients, the researchers did find some commonalities between them. Specifically, Shirvalkar and his team found that in all patients, low-frequency power—specifically delta waves between 1 and 4 hertz—increased in lower OFC areas. These areas were also located on the side of the brain opposite to, or contralateral to, the side of the body where the patients were experiencing pain. “The top ten features in models fit to predict pain from individual patients were different, but the main pattern that emerged—and this was surprising—was the contralateral OFC” says Shirvalkar. “These patients all had pain on only one part of their body, which presented us with an opportunity. We didn’t know much about brain laterality when it comes to pain. It’s kind of a nebulous thing.”
Kate Nicholson, founder and executive director of the National Pain Advocacy Center, says that this research may lead to better quantification of pain and treatment for those suffering from central pain disorder and other conditions that cause chronic pain. “The search for an objective measure of pain phenotypes is the holy grail. Alongside subjective reporting, this would assist providers in treating pain,” says Nicholson. “Even though this study only looked at four people, and at a very specific kind of neuropathic pain, it’s certainly interesting what they’ve found. It’s super exciting, but it’s stage one.”
Moving forward, Shirvalkar plans to incorporate what they’ve found into better stimulation treatments for chronic pain. “This study’s just scratching the surface,” says Shirvalkar. “In the future we could have a stimulator that changes frequencies and location of stimulation. It’s a moving target: When you stimulate, you change the brain’s states. You’re trying to track natural fluctuations in the brain and then trying to know what your stimulation is doing, and then trying to integrate those into a common model.”
- How Do Neural Implants Work? - IEEE Spectrum ›
- Nerve Coolers: Pain Relief Without Drugs - IEEE Spectrum ›
- Flexible Optogenetics Implants Hack the Sense of Pain - IEEE ... ›
Michael Nolan is a writer and reporter covering developments in neuroscience, neurotechnology, biometric systems and data privacy. Before that, he spent nearly a decade wrangling biomedical data for a number of labs in academia and industry. Before that he received a masters degree in electrical engineering from the University of Rochester.