A Temporary Tattoo That Senses Through Your Skin
The Biostamp can replace today’s clunky biomedical sensors

I turn the key to start the little Ford SUV I’ve rented for my visit to the University of Illinois at Urbana-Champaign, and a message flashes briefly on the dash: “Tire pressure low.” I ignore it. My own car is 12 years old; I’m not accustomed to a car that monitors its own health. Turns out, though, that the little Ford wasn’t kidding. The next morning I find the car has a flat tire.
Modern cars are laden with sensors that constantly monitor the vehicle’s vitals and indicate, for example, when a filter needs replacing or whether the air bag is working. Electronics diagnose failures after they happen and even predict problems that are imminent. Wouldn’t it be great if we could monitor our bodies in much the same way?That very idea, ironically enough, is what has brought me to Illinois. I’m here to see John Rogers, a materials science professor at the University of Illinois with a bold vision: Someday, he believes, we will all have sensors on our bodies that send information to a mobile phone, similar to the way a car’s sensors feed the vehicle’s computer.
How it works
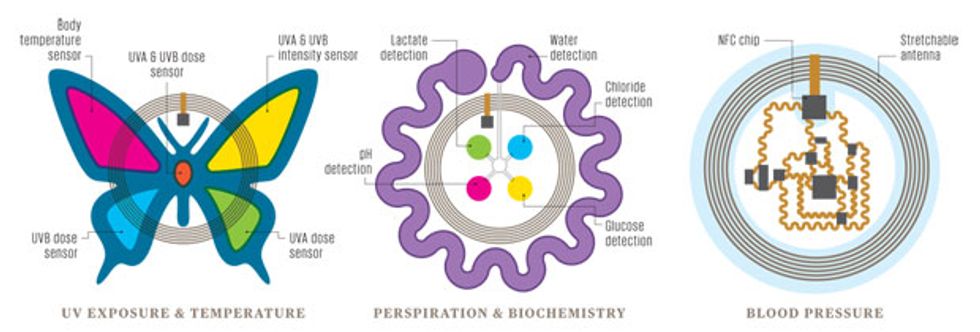
Inside the Biostamp
While all biostamps have a few common characteristics—they stretch like skin, include flexible circuits, and can be powered wirelessly— different functions require different sensors. The butterfly sensor on the left is designed to monitor exposure to the sun’s ultraviolet (UV) rays, the center sensor uses sensitive dyes to detect chemicals in sweat, and the sensor at the right uses electronic circuitry to measure blood pressure.
We’ve already taken the first steps in this direction. Many of us now wear fitness bands that track our activity and heart rate and assume we’re sleeping if we don’t move for a while. But most of these bands aren’t exactly chic or unobtrusive, so even the more die-hard among us take them off sometimes. And the information they dispense is interesting but hardly vital: They can’t detect signs that you’re getting sick or tell your doctors anything they need to know, much less replace an office visit.
But there’s no reason why they can’t, says Rogers. Think about your last medical exam. Your doctor checked your pulse, your temperature, your blood pressure, and maybe your blood oxygen. If any anomalies showed up, you may have been sent for further tests—perhaps an electrocardiogram for your heart, a blood test to check for diabetes, electromyography if you were having muscle weakness, or possibly even a polysomnogram at a sleep lab to check for apnea. All of these tests require specialized and costly equipment, trained medical technicians, or invasive pokes.
These tests—and more—could be accomplished by means of sensors so light, durable, and comfortable that you could wear them on your body for weeks at a time. It’s no distant dream: At press time, several sensors developed by members of Rogers’s research team had entered or were about to enter clinical trials in the United States and Europe, and the first commercial versions were expected to become available by the end of this year.
Rogers says these sensors are so much like skin that you don’t notice you’re wearing them—and I didn’t have to take his word for it. I wore one on my inner forearm for more than a week. This version was a test unit that simply transmitted a greeting when triggered by an Android smartphone; units with biosensors haven’t yet been made available to journalists.
Simple though it was, my sensor delighted me. It clung unobtrusively and tenaciously to my arm as I went about my life—showering, sleeping, and exercising. It also got me thinking about how future versions of these sensors are going to make our lives better—not a decade from now but within a couple of years, Rogers promises.
The Illinois team isn’t the only one trying to make skinlike electronics. Takao Someya is leading a group at the University of Tokyo that’s working to develop electronic skin made of organic semiconductors and carbon nanotubes. Zhenan Bao at Stanford is also working with organic semiconductors to develop an electronic film that would be as sensitive as human skin and could be applied over robotic limbs. And researchers at the University of California, San Diego, are developing inks that would allow scientists to draw sensors directly onto the skin.
But Rogers’s skinlike sensors are poised to be the first to get out of the lab and onto our bodies. In 2008, Rogers teamed with Roozbeh Ghaffari to start a company called MC10, in Cambridge, Mass., to turn his group’s research on stretchable electronics into commercial health care products. MC10 today has about 60 full-time employees, US $60 million in venture capital and corporate investment, and one product on the market: the Checklight, a skullcap for precisely measuring accelerations during athletes’ head impacts. It’s not a skinlike sensor patch, but it does bend to conform to the shape of a body part. (Rogers serves on the board of MC10 and helps plan the company’s research and technology efforts with Ghaffari, who is now MC10’s chief technology officer.)
MC10 started making the first skin patches—the company calls them Biostamps—in late 2012. Most of these early units were used for internal development or codevelopment efforts with partners. MC10 began developing a new generation of the technology in late 2014; most of these Biostamps are now going to medical researchers for use in clinical trials. Consumer-wellness Biostamps are also being developed for companies targeting their own special niches. A cosmetics company may package a sun-monitoring Biostamp with sunscreen, for example, or a pharmaceutical company could include motion- and temperature-monitoring Biostamps with a package of medication.
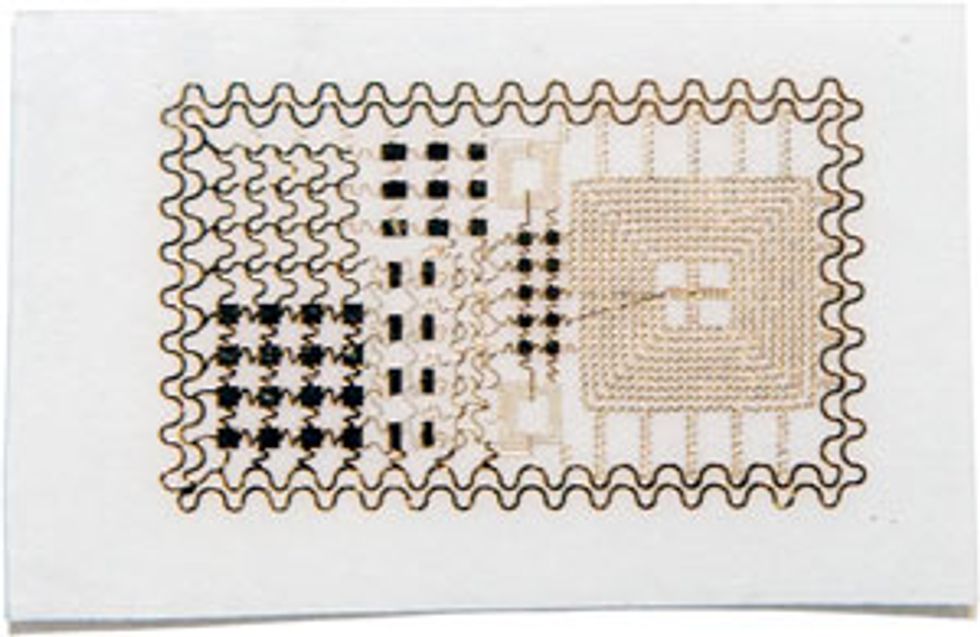
The basic Biostamp is a thin sticker about the size of a British ten pence or an American quarter. It looks like a temporary tattoo a child might get at a birthday party, but because it has been designed to be mechanically similar to skin, it can’t really be felt by the wearer once it’s applied. A Biostamp can contain hundreds of thousands of transistors, as well as resistors, LEDs, and a radio-frequency antenna. It’s waterproof and breathable, and it costs just tens of cents when manufactured in quantity. It can be worn for a week or so, before the normal shedding of skin cells begins to force the thin substrate to peel from the skin, like an early-season sunburn.
A Biostamp is built out of stretchable circuits supported by an extremely thin sheet of rubber. To make these circuits, Rogers and his colleagues in Illinois start by fabricating their transistors, diodes, capacitors, and other electronic devices on wafers of any common semiconductor material. They typically use silicon but could also use gallium arsenide or gallium nitride. These are not ordinary semiconductor wafers; they’re kind of like the Oreo cookie of semiconductor wafers. They have a thin top layer of semiconductor material, a thicker bottom layer of the same material that acts as a rigid support during manufacture, and a sacrificial layer of a different material in between. In the case of a silicon wafer, this sacrificial layer is silicon dioxide. After the device manufacture is complete, a chemical bath eats away that central layer and frees the thin top layer.
Then a stamp made of soft silicone presses onto the wafer. Raised areas on the stamp lift away selected electronic devices in the same way a rubber stamp picks up ink from a stamp pad. After picking up the devices, the silicone stamp deposits them onto a temporary substrate, usually a plastic-coated glass plate. This plate then goes through a standard photolithography process that connects the devices with copper conductors in the form of serpentine coils, which make the connections stretchable.
The next step is to transfer the interconnected devices from the plastic-coated glass onto what will go to the consumer—a thin sheet of rubber already attached to a plastic backing sheet, with a layer of adhesive in between. To do this, a machine pushes the rubber against the array of devices and coils that are still clinging to the plastic-coated glass. A final chemical bath dissolves the plastic between the electronic circuits and the glass, leaving the circuits attached to the rubber. And the last step happens when the Biostamp gets into the hands of the user—who exposes the adhesive and sticks the rubber-backed electronics onto the skin.
In many Biostamps, all the electronics are created using this process. In some cases, however, a Biostamp design incorporates a microprocessor without packaging, which the researchers thin down to 5 to 10 micrometers. A few more micrometers of flexible resin cover the circuitry to protect it from water. For now, though, most Biostamps don’t have full-blown microprocessors on board. Most of those now being tested simply gather data and transmit it; analysis happens elsewhere, generally on a smartphone or tablet.
A Biostamp powers itself by harvesting energy from near-field communication (NFC) radio signals, typically from the wearer’s cellphone. It communicates with the phone the same way. NFC, which sends data at 13.56 megahertz, is a feature of almost all current-model smartphones, which use it for wireless-payment schemes. At the moment the stamps work only with Android phones, but the hardware is compatible with the type of NFC technology on the newer iPhones.

The stamp converts the RF energy picked up by an antenna to electrical energy by means of an inductive coil. A Biostamp can generate tens of milliwatts of power when within a meter or so of a phone transmitting an NFC signal. For longer-distance power gathering, a Biostamp can be built to receive radio signals at frequencies between 1 and 2.5 gigahertz from a transmitter up to several meters away.
The current tattoo-like versions of the technology don’t store energy, although Rogers’s group and MC10 have already built and tested stretchable batteries and supercapacitors. But in a hospital room, say, with an NFC transmitter under the bed or a longer-distance RF transmitter in a corner, Biostamps can operate continuously and indefinitely.
Right now, Rogers and his students are evaluating stretchable sensors that measure body temperature, monitor exposure to ultraviolet light, and check pulse and blood-oxygen levels. They’re also developing sensors that can track changes in blood pressure, analyze sweat, and obtain signals from the brain or heart for use in electroencephalograms and electrocardiograms. All of these sensors, Rogers says, are intended to make measurements with enough accuracy to be useful in medical settings—a much higher standard than that needed to build a typical consumer wearable.
Achieving that standard in a thin and stretchable device has compelled Rogers and his team to rethink how medical measurements are done. Consider blood pressure: It’s usually measured by placing a cuff around a patient’s arm and inflating the cuff until it cuts off blood flow. Then the cuff gradually deflates until blood starts flowing through the vessels in the arm again; that gives the systolic reading, a measure of the pressure during the contraction of the heart. The cuff continues to deflate until the doctor can no longer detect the sound of the blood flowing. This produces the diastolic reading, the pressure when the heart muscle is relaxed, between beats.
A tiny Biostamp can’t cut off blood flow. It can, however, measure the pulse at two points, just a centimeter or so apart. With this information, a smartphone can calculate a physiological indicator called pulse-wave velocity, which varies as blood pressure changes. So researchers Tony Banks, SeungMin Lee, and Matt Pharr in Rogers’s group are developing two types of Biostamp pulse detectors. One uses light; it alternately flashes a red and an infrared LED and uses a photodetector to pick up the light reflected from the skin beneath the Biostamp. Because deoxygenated blood absorbs more red light and oxygenated blood absorbs more of the infrared, fluctuations in those levels create a waveform that represents the heartbeat. That’s basically how the newest fitness bands detect a pulse, though the Biostamp version can get a more stable signal because the skin doesn’t shift under it. The other type of pulse detector under development uses piezoelectric strain sensors to monitor the stretching and relaxing of the patch as it reacts to the blood coursing through the vessels under it. In this scheme, greater stretch translates to higher pressure.
In either case, these measurements of pulse converted to pulse-wave velocity tell the wearer only how blood pressure is changing—they don’t give the baseline blood pressure reading. But for patients whose blood pressure needs to be monitored closely, simply keeping track of variations is vital. Instead of seeing a nurse daily for a blood pressure check or using bulky home monitoring equipment, the patient would put on a new Biostamp every week or two, get it calibrated, and just scan the stamp with a phone to take a reading that could be sent automatically to the doctor.
Another promising Biostamp version will monitor sweat. Daeshik Kang and Aheyon Koh, postdocs in Rogers’s group who are working with research staff member Banks, have built a Biostamp with microfluidic channels that wick sweat along a calibrated path. Chemically sensitive dye on the path changes color as the sweat hits it, while other dyes on the patch change color in response to glucose, lactic acid, chloride, and sodium. When scanned with a smartphone, circuitry on the patch activates a phone app that analyzes the color changes and offers suggestions, such as “Time to hydrate,” or, for a woman being monitored for pregnancy-onset diabetes, “Time to visit the doctor.” MC10 researchers, in collaboration with Rogers’s group, are also investigating possible ways to use this sweat-derived data for monitoring cardiac health.
These chemically sensitive Biostamps, unlike fully electronic wearable sweat monitors being developed elsewhere, aren’t reusable; at the end of your workout, race, or stress test, when you’ve stopped sweating, you won’t be able to reset the patch, so you’ll have to throw it out. They’ll be so cheap, though, that you won’t care.
Rogers is confident that Biostamps will become staples in hospitals. An initial trial began this year in the neonatal intensive care unit at Carle Foundation Hospital, in Urbana, Ill., where doctors are using Biostamps to monitor temperature and other vital signs of newborns. The doctors stick Biostamps on an arm, a leg, the forehead, and the chest of each infant, while an NFC antenna under each incubator powers the devices.
As word of his Biostamps gets out, Rogers and MC10 have been fielding requests not just from doctors and trainers but also from government officials and business executives. Many discover his work by reading one of the hundred-plus papers that have appeared in scientific journals in recent years.
“I read his paper in Science four years ago,” says Guive Balooch, global vice president in charge of new technologies for L’Oréal, the hair-care and cosmetics giant. “We went to him because measuring the skin and understanding changes over time can help us identify and test products.”
L’Oréal is now working with a Biostamp group on a skin hydration sensor that monitors how heat travels across the skin under the patch. A device on the patch creates a tiny burst of heat that is detected by a temperature sensor on the same patch. L’Oréal is hoping to eventually use this information to test the efficacy of its products; the patch could track changes in hydration as people use its products over time and more general changes as the skin ages. Researchers at the company have already done one trial with 20 subjects, each wearing six Biostamps. The initial study simply established correlations among hydration, temperature, skin thickness, and the travel of heat through the skin. Within 5 to 10 years, Balooch expects, the technology will do more. “I would love to see a beauty patch on someone’s body give them skin-care recommendations,” he says. L’Oréal is also funding research on Biostamps that measure UV exposure and signal when it’s time to reapply sunscreen.
In my week-plus of wearing a stripped-down Biostamp, I found myself eager to roll up my sleeve and show it off. After seeing countless demos in Rogers’s lab of potential applications, and recalling the L’Oréal research in particular, I even became annoyed about having to do things the usual way. Sitting in the sun one hot afternoon, I debated whether to reapply sunscreen and, looking at the patch on my wrist, I thought, “You could tell me this, you know.”
Later that same week, feeling like I was coming down with a cold, I went hunting for a thermometer. Again I glared at my Biostamp and wished it were the temperature-sensor one I’d seen demonstrated in Illinois. And the Fitbit Flex I’ve been wearing for more than a year and once thought was so sleek? It looks huge and clunky to me now.
Other researchers are studying the possibility of using temperature-sensing Biostamps to measure mental stress in air traffic controllers (the tougher the mental task, the cooler the temperature of the hand) and using heat-generating Biostamps to push drugs through the skin. In a clinical trial at Northwestern University’s medical school, in Chicago, research teams have tested Biostamps that measure both temperature and heat flow in tissue to monitor wound healing. And Biostamps that adhere behind the ear to measure the electrical activity of the brain for sleep studies were to go into clinical trials this spring at Carle Foundation Hospital. This method would be far less cumbersome than the wired sensors typically used now.
Of course, any device that gathers health data must ensure patient privacy, so any apps that use Biostamp data will have to comply with the security requirements of the Health Insurance Portability and Accountability Act in the United States and analogous privacy regulations around the world. But unlike other data-gathering devices, Biostamps also have the potential of making health information more secure. Because it can’t be removed without being destroyed, a Biostamp can be a physical key used to control access to data, whether in a patient’s smartphone or at a nurse’s workstation.
The early tests of different Biostamps have shown encouraging results, but they were labor intensive, requiring Rogers’s group to design each sensor from scratch. So while medical researchers have plenty of ideas about how a proposed sensor could help patients, creating it can take months or even years. And the stamps’ limited memory and external power restrict the kinds of applications that are feasible.
To address this issue, Rogers and MC10 have developed a version of the Biostamp that’s larger (about the size and shape of a Band-Aid, though slightly thicker), reusable, and equipped with a variety of sensors, batteries, and memory. The patch can be placed on a variety of spots on the body, and the signals it collects can be analyzed by smartphone or tablet apps. This reusable version includes off-the-shelf dies (chips stripped of their packages) for NFC or Bluetooth Low Energy communications and multiple sensors, small squares of lithium-ion batteries, and, for linking these components together, the kind of serpentine coils that Rogers invented for the stretchable circuits used in the tattoo-like Biostamp.
Researchers have started using these patches in clinical trials and as a platform for the development of new applications, some of which may migrate to the smaller skinlike Biostamp. MC10 is planning to market these larger, reusable Biostamps as a competitor to today’s health-tracking devices in 2016. This gadget will be functional as long as its batteries are able to hold a charge. Depending on how often you end up recharging it, that’s likely to be two years or more.
With skinlike wearable electronics on the verge of commercialization, Rogers is turning his attention from what can be sensed from outside the body to electronics that can be worn on the inside. He’s collaborating with researchers at the University of Pennsylvania on an array of some 400 electrodes that can be draped across brain tissue to map activity that signals an epileptic seizure. The researchers have conducted trials with cats and will begin primate trials soon.
Other researchers are using excised hearts from organ donors to test Biostamps that could be laminated directly onto the heart’s surface. This type of sensor—and, eventually, a mesh one that wraps completely around the heart and harvests energy from its beating—would give detailed information about arrhythmias and could provide finer control for pacemakers, which currently monitor a single point in the organ.
Lately, Rogers has begun to mull over a new challenge. Some parts of the body, like the brain and the heart, have twists and turns and crevices that cry out for a 3-D solution rather than his 2-D one. “We’d like to be able to not only transform circuits from planar wafers into thin, soft sheets that can be wrapped onto complex surfaces,” he says, “but to induce them to self-assemble into open, 3-D formats, with filaments and arrays of interconnected structures that completely permeate a biological system. That capability would bring us into an entirely new realm of biointegration.”
Such an application remains more than a decade away. But Rogers’s nearer-term vision is pretty compelling, too. This enthusiastic inventor fully expects that within a decade, nearly everyone in the developed world will be wearing one or more Biostamps, at least some of the time.
Fast-forward to 2025. At this point, if Rogers’s and MC10’s dreams come true, a baby in a developed country will be tagged with several Biostamps at birth. One, on a wrist or ankle, will serve as a high-tech hospital bracelet—and will be far harder to lose than today’s plastic cuffs. Others on the torso or arm will allow nurses to perform quick scans of temperature, oxygen saturation, and pulse without disturbing a sleeping infant. The mother of that baby will also wear a few Biostamps, allowing nurses to monitor her vital signs as she recovers; no longer will an automatically inflating blood pressure cuff wake up an exhausted new mom.
In that same hospital, cardiac patients will wear vital-sign Biostamps plus two more on their ankles to check for swelling, an early sign of heart failure. After the cardiac patients are discharged, the stamps will continue that monitoring at home. Even the nurses will wear Biostamps, to enable them to unlock doors and log on to their computers; these devices will provide far more security than scan cards or pass codes.
Outside, the joggers running past the hospital will wear Biostamps to monitor their progress toward fitness goals. Though a typical jogger probably isn’t suffering from serious medical conditions, the fitness Biostamps will spot early signs of cardiac problems or movement disorders like Parkinson’s disease and suggest that the wearer check in with a doctor.
Meanwhile, passengers lining up to board a cruise ship in the nearby harbor will be busily adding one more Biostamp to the ones they usually wear. Designed with the cruise ship logo on it, it will act as a secure ID to permit them to board the ship. It will also unlock their cabin doors, allow them to charge their drinks at the bar, and even monitor their sun exposure over the course of the week and warn them when it’s time to reapply sunscreen.
This coming ubiquity of the Biostamp may be hard to imagine, given that most people haven’t even seen one yet. But technology sometimes surprises us. iPhones and Androids were still on the drawing board 10 years ago, and now we rely on them for constant access to information about the outside world. A decade from now, if Rogers succeeds in ushering in the world of the Biostamp, we’ll know just as much about our internal worlds.
This article originally appeared in print as “Giving Your Body a ‘Check Engine’ Light.”