In a significant breakthrough that could reshape the landscape of battery technology, researchers at the University of Liverpool have unveiled a novel solid-electrolyte material capable of conducting lithium ions at a speed equal to that of liquid electrolytes.
Solid electrolytes offer a key benefit over the liquid variety—they don’t blow up. Of course, until now that improved safety came at the price of reduced performance. Specifically, solid electrolytes are typically worse at conducting lithium ions through their material.
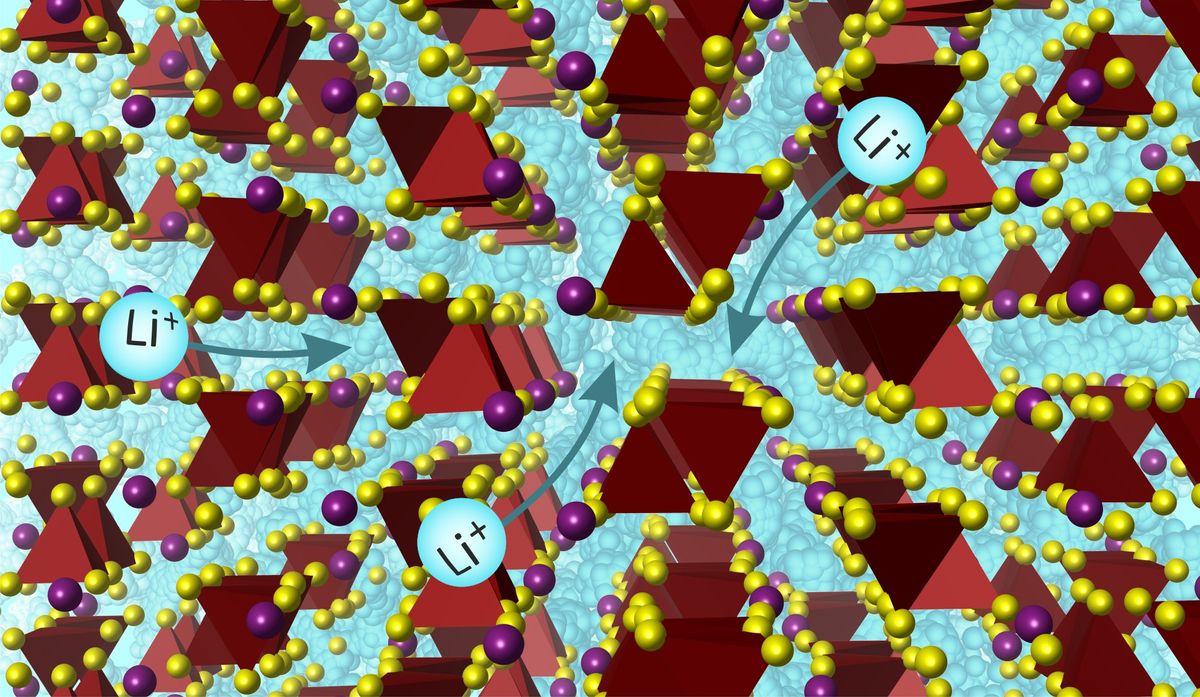
Now, the Liverpool researchers have addressed the ion-transport issue with a new solid electrolyte made out of lithium, silicon, sulfur, and iodine that takes the shape of a three-dimensional crystal structure. The researchers published their work in Science on 16 February.
This structure offers 15 different pathways for lithium ions to navigate through the material. These pathways are known as coordination environments, which refer to the way positively charged ions (cations) are surrounded by and interact with neighboring atoms or molecules.
The number of pathways marks a substantial departure from the current state-of-the-art lithium solid electrolytes, which typically feature only three or four different lithium environments within their crystal structures.
“This material is not directly competing with established solid electrolytes. The aim of this research is to pioneer a new direction in solid electrolyte discovery, focusing on enhancing lithium mobility and overall battery performance.” —Matthew Rosseinsky, University of Liverpool
“The large number of environments provides a very large number of pathways for lithium to move through the crystal structure,” said Matthew Rosseinsky, a professor at the University of Liverpool and one of the paper’s coauthors. “In addition, there is a high degree of disorder in the lithium environments within the material. For ion transport in solids, the more disorder there is, the better.”
The abundance of diverse pathways for lithium movement within the crystal lattice allows for efficient ion conductivity, according to Rosseinsky. Unlike traditional solid electrolytes that favor fewer and more similar pathways, this new material combines a higher density structure with a diverse set of pathways, providing lithium ions with a multitude of options for movement. This structural diversity reduces the likelihood of ion trapping and improves overall conductivity.
In their study of the material, the researchers confirmed that the exceptional electrical conductivity of the material was comparable to that of liquid electrolytes. Additionally, the material exhibits a low activation energy, indicating minimal energy barriers for lithium-ion movement. The researchers believe that these promising results position the new material as a viable candidate for use as a solid electrolyte in next-generation battery technologies.
In practical battery applications, the new material demonstrates impressive performance features, according to Rosseinsky. Operating as the electrolyte in an all-solid-state cell configuration alongside a lithium cobalt oxide cathode and lithium metal anode, the new material has better energy density, power density, and cycle life compared with traditional liquid electrolytes.
“This material is not directly competing with established solid electrolytes. The aim of this research is to pioneer a new direction in solid electrolyte discovery, focusing on enhancing lithium mobility and overall battery performance,” said Rosseinsky.
Rosseinsky also emphasizes that the composition of the new solid electrolyte consists of nontoxic, earth-abundant elements, underscoring its potential environmental benefits.
“With lithium, silicon, sulfur, and iodine being readily available and nontoxic, the material aligns with efforts towards sustainability and resource-use efficiency within the battery industry,” Rosseinsky said. “Furthermore, the use of earth-abundant elements supports initiatives for battery recycling, minimizing environmental impact and promoting a circular economy.”
The significance of this advance extends beyond just battery research. The researchers incorporated AI models into their research, which allowed them to broaden their search for possible high-performance materials. In the research and development process, AI facilitated the identification of promising chemical compositions, guiding researchers toward materials with unique structural characteristics conducive to ion transport.
However, Rosseinsky notes that “the AI was a useful tool for the researchers. But the process was at all times directed by the researchers themselves based on their understanding, and drew on a broad variety of methods, most of which did not use AI.”
Rosseinsky concedes that challenges remain in the widespread adoption of the material. The material’s instability in air and moisture presents a significant obstacle in manufacturing, requiring controlled environments during battery assembly to maintain stability. Additionally, while the new material offers promising performance features, further research is needed to optimize its synthesis, scalability, and compatibility with existing battery technologies.
- Ion Storage Systems Says Its Ceramic Electrolyte Could Be a Gamechanger for Solid-State Batteries ›
- To Make Solid Electrolytes, Start With a Liquid ›
Dexter Johnson is a contributing editor at IEEE Spectrum, with a focus on nanotechnology.