23 April 2012—With a zap of electricity and a flash of light, organic molecules pull together to form tiny conduits that conduct electricity as efficiently as metals do, researchers in France have found. The scientists say that the conductive organic nanowires could be useful for making low-cost flexible electronic circuits.
Flexible electronic devices, such as light-emitting diodes, thin-film transistors, and solar cells, all rely on films of organic molecules with semiconducting properties. The advantage of such films is that they can be turned into ink and printed on pliable substrates. The wiring that strings the devices into a circuit, however, is metal, a material that is difficult to process, expensive, and brittle, says Nicolas Giuseppone, a chemistry professor at the University of Strasbourg, in France. As devices and circuits shrink, using organic interconnects would be easier than building metal ones and would lead to truly flexible low-cost electronics. “Ideally, you want the metallic part of the organic circuit to be made of a organic material [that conducts like metal], which will make it lighter, softer, and more cost-effective,” he says.
People have been exploring carbon nanotubes as a substitute for metal, because they are highly conductive, flexible, and cheap. But so far, no one has devised a good way to make large pure batches of conductive nanotubes: Today’s methods result in mixed batches of conducting and semiconducting nanotubes. It is also difficult to place nanotubes in precise locations within a circuit.
Conductive polymers also have problems. Adding impurities to, or “doping,” semiconducting polymers, such as polyacetylene and polyaniline, makes them conduct the way metal does. However, because these materials are expensive to process, few practical large-scale applications have been found. Specifically, they are not easily dissolved into a solution, which makes it difficult to create uniform films or build complex structures for making devices.
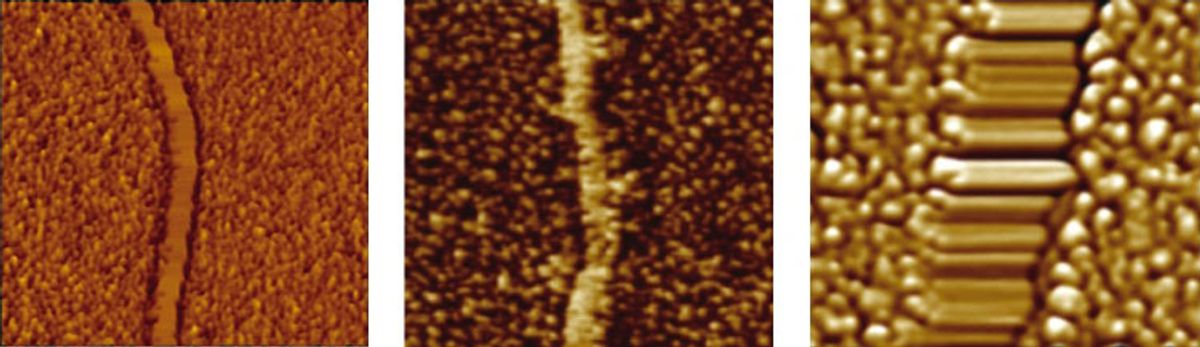
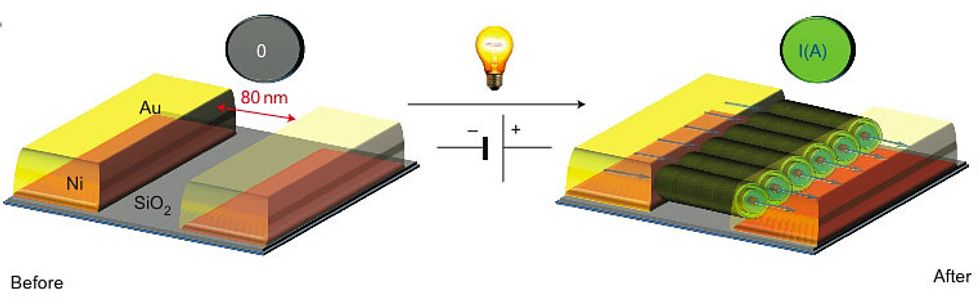
Giuseppone, Bernard Doudin, and their colleagues have a different solution: a small organic molecule called triarylamine. They took a solution of the molecule and placed drops of it on top of gold and nickel electrodes on a silicon substrate. They then applied an electric field between the electrodes and shone white light on it for 10 seconds. The organic molecules lined up in a row, forming nanowires parallel to the electric field that spanned the 80-nanometer gap between the electrodes.
“We can self-construct organic [conductive] wires where we want them on a surface,” Giuseppone says. “For good conductivity, you need a really good molecular organization of the material, like a crystal.” But more-organized molecules in a material also make it more rigid and harder to process. “Here we have both,” he says. “An organization between crystal and plastic, sufficient for good conductivity, but also a soft chemical structure to allow processing.”
The conductivity of the organic nanowires is greater than 5000 siemens per meter, which is about a hundredth that of bulk copper. But at the nanometer scale, the organic wires can carry as much current per unit area as copper wires. The key characteristic of metallic conductivity, however, says Giuseppone, is that a material’s resistance should decrease with a drop in temperature, and the nanowires display this behavior. The researchers detailed their results this week in Nature Chemistry.
“This is a truly interesting result,” says Christopher Gorman, a chemistry professor at North Carolina State University, in Raleigh. “The ability to self-assemble a wire that is this conductive is unprecedented and suggests a novel way to build electronics from the bottom up.”
Self-assembled stacks of organic molecules are typically thought to have low conductivities, says Miklos Kertesz, a chemistry professor at Georgetown University, in Washington, D.C. “These nanowires bridge two electrodes showing unusual and remarkable high metal-like conductivities,” Kertesz says. While the practical implications of the work might not be immediately clear, it could lead to new types of transparent conductors, nanoscale transistors and connectors, or other novel applications, he adds. “What’s important is to bring together properties in materials that have never been seen before, and then that becomes an enabler of technology,” he says.
About the Author
Prachi Patel is a contributing editor to IEEE Spectrum and a freelance journalist in Pittsburgh. In February 2012 she reported on a new route to embedding electronics inside optical fibers.