Custom Cardiology: A Virtual Heart for Every Patient
Personalized computer models will let cardiologists test life-saving interventions

A poet might say that each human being’s heart is a unique mystery. Those of us working in the brand new field of computational medicine, however, can now model each of those unique hearts with marvelous accuracy and reveal their secrets. In my laboratory at Johns Hopkins University, my team creates computer models to simulate individual patients’ hearts, which can help cardiologists carry out life-saving treatments. Such models may soon transform medicine, ushering in a new kind of personalized health care with radically improved outcomes.
Biomedical engineers have learned how to use numerical models to generate increasingly sophisticated “virtual organs” over the past decade, and rapid developments in cardiac simulation have made the virtual heart the most complete model of all. It’s a complex replica, as it must mimic the heart’s workings at the molecular scale, through the cellular scale, and up to the level of the whole organ, where muscle tissue expands and contracts with every heartbeat. What’s more, the modeling at these different scales must be tightly integrated to accurately render the constant feedback interactions that govern the functions of the heart.
Such models have already proved their value for basic cardiac research, allowing scientists to plug in experimental data and study what goes on in both normal and diseased hearts. Now, virtual hearts are poised to deliver breakthroughs at the bedside.
Starting with a patient’s MRI scans, specialists in computational cardiology can create a personalized model of the patient’s heart to study his or her unique ailment. Doctors can then poke and prod the computerized organ in ways that simply aren’t possible with a flesh-and-blood heart. With these models at their disposal, cardiologists should be able to improve therapies, minimize the invasiveness of diagnostic procedures, and reduce health-care costs. While this simulation-based medicine is still in the experimental stages, I believe upcoming clinical trials will show the real value of virtual hearts.
To grasp the vital importance of this technology, you have to understand today’s standard of cardiac care. So imagine a patient—let’s call him Jim—who has survived a serious heart attack. It was a terrifying experience. He fell down, clutching his chest, fearing he was about to expire. But he was rushed to the emergency room, where doctors took swift action to restore the flow of oxygen-rich blood to his heart muscle. Jim is alive—but not quite as alive as he used to be. Some of the heart muscle, which was deprived of oxygen during the crisis, has died. The resulting patches of scar tissue can interfere with the electrical signals that propagate through the heart muscle, thus disrupting the contractions that should pump blood through the body in a steady rhythm.
Making the Mock-up
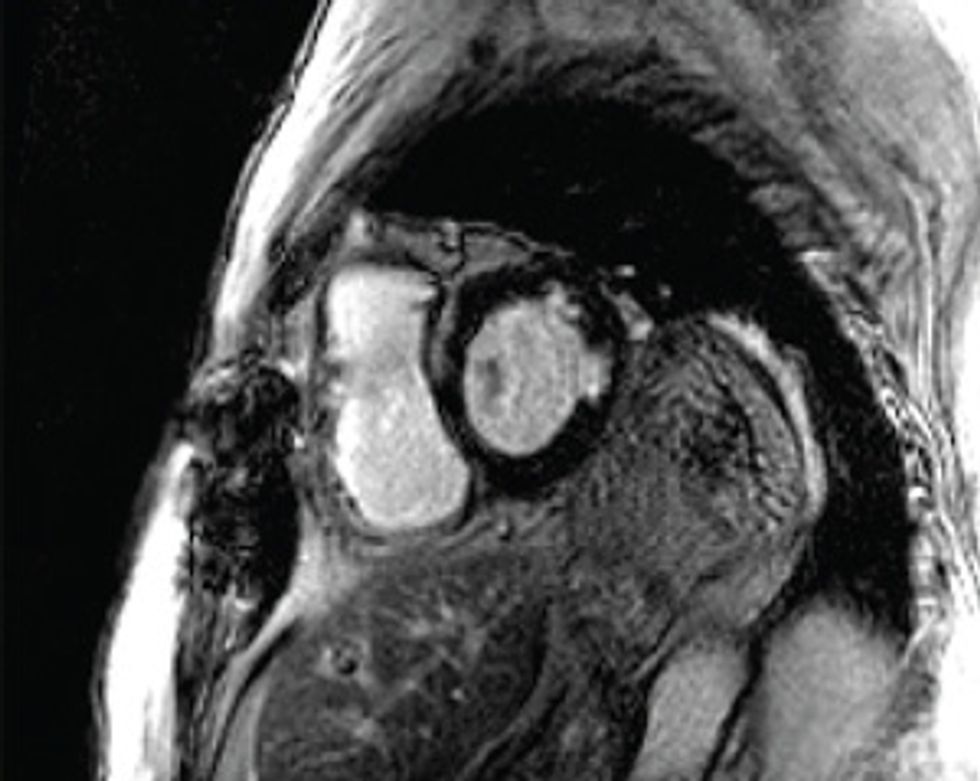
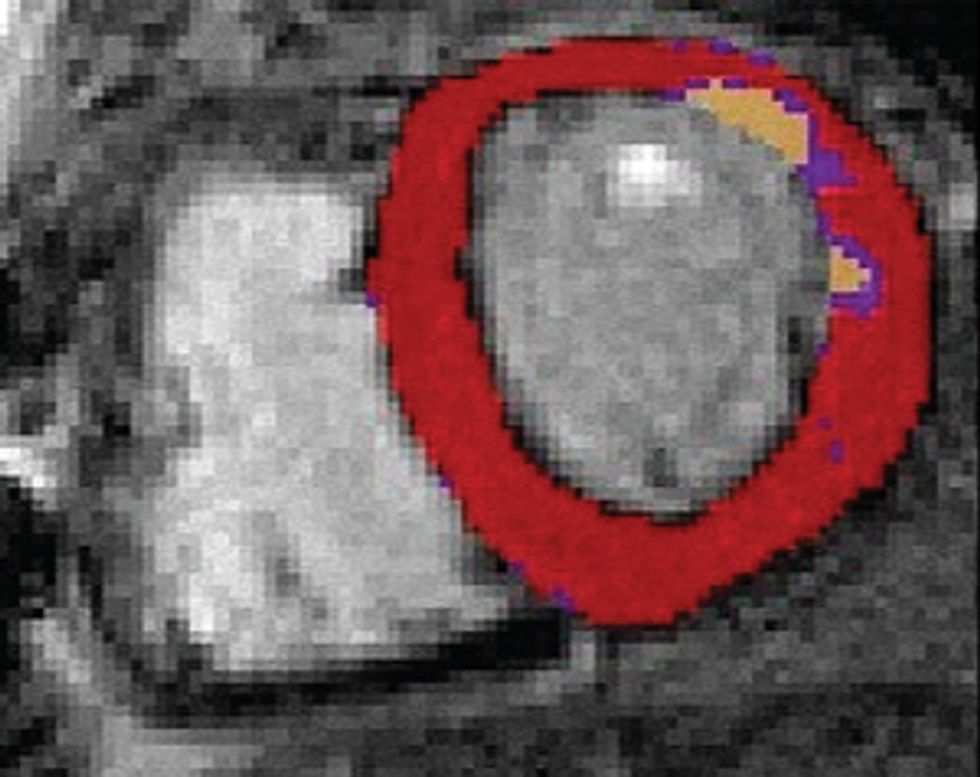
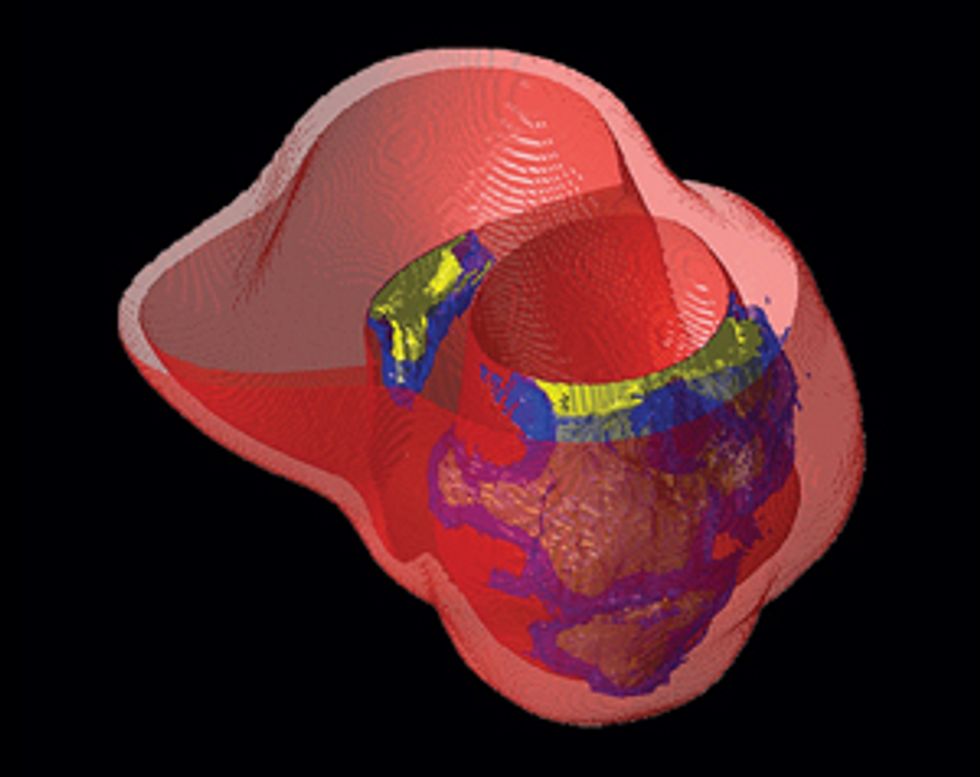
Here’s how to create a personalized model of the heart of a patient who has suffered a heart attack. It’s necessary to map the pattern of scar tissue and the orientation of muscle fibers, because these factors determine how electrical signals move through the cardiac tissue to produce a regular heartbeat.

If Jim develops an irregular heartbeat, called an arrhythmia, he may be in serious danger of cardiac arrest. His doctors must assess whether he’s at high risk of developing this life-threatening condition and decide whether to implant a defibrillator in his chest. Just like external defibrillators used in ambulances and emergency rooms, an implanted device shocks the heart back into normal rhythm if arrhythmia develops, thus saving the patient’s life. To keep an at-risk patient like Jim from ending up in the ER, doctors may insert the internal device as a precautionary measure.
How can doctors judge whether or not to implant such a device in Jim? It’s a big decision, because they don’t want to needlessly put him through this invasive procedure and expose him to the possible complications that come with the defibrillator. Currently, cardiologists make their decision based on the patient’s ejection fraction—the proportion of blood that is pumped out of the heart with every beat. If this number is below 35 percent, then doctors advise the patient to undergo the implantation procedure. Lots of patients are getting implants based on this strategy, but in the first year after the procedure only 5 percent will go on to develop ventricular arrhythmias and receive a necessary shock. In other words, 20 devices are implanted for every one life saved.
It’s clear that many patients are needlessly risking surgical complications, infections, and device breakdowns. The defibrillators aren’t perfect, and the electrodes that monitor the heart can malfunction, triggering an unnecessary shock. And a shock is a serious event—it can feel like getting kicked in the chest by a horse. Patients sometimes lose consciousness, which could prove deadly if they’re driving, for example, or soaking in the tub. Most important, the ejection fraction isn’t a good predictor of arrhythmia; it misses many at-risk patients. Many patients who don’t fit the current criteria for an implanted defibrillator go on to die of sudden cardiac arrest, often in the prime of their lives.
My colleagues and I are now testing whether we can use patient-specific heart models to make better predictions of a person’s risk of developing a life-threatening arrhythmia, and hence his or her need for an implanted defibrillator. To do that, we run simulations on the patient’s virtual heart to assess how prone it is to arrhythmia. We can do risky things to the virtual heart that physicians are reluctant to do to a live patient—such as generate small electric pulses in different locations and then watch to see whether arrhythmia develops.
Our first retrospective study at Johns Hopkins, in Baltimore, was promising. We created heart models for about 40 patients who had suffered heart attacks and received implanted defibrillators, and we predicted which of them would develop arrhythmias in the five years after surgery. Then we compared our forecast with the real data from that group of patients, revealing which of them had indeed received shocks from their devices to terminate arrhythmia episodes. With our virtual heart simulations, we correctly identified those at-risk patients 85 percent of the time over that five-year time span. In comparison, the accuracy of standard predictions for this group based on ejection fraction was only 51 percent over the same period of time.
To validate our method and get this test to the clinic, our team is now creating individualized virtual hearts for post-heart-attack patients who have an ejection fraction greater than 35 percent (who therefore don’t qualify for an implanted device). Clinical recommendations for these patients are sparse, but we can run our simulations and make our predictions. If we discern patients in that group who are at high risk for a lethal arrhythmia, we can recommend that they receive implants despite their relatively high ejection fractions. We’re working toward a day when cardiologists routinely order these virtual tests as a noninvasive way of screening their patients and gauging their risk of sudden cardiac death.
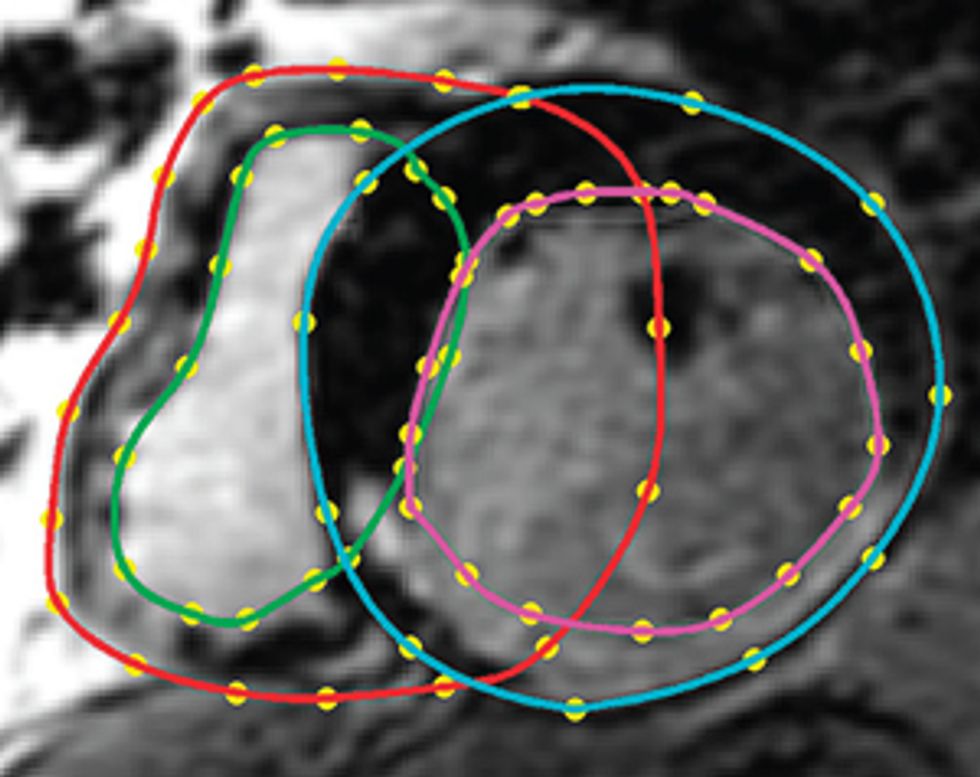
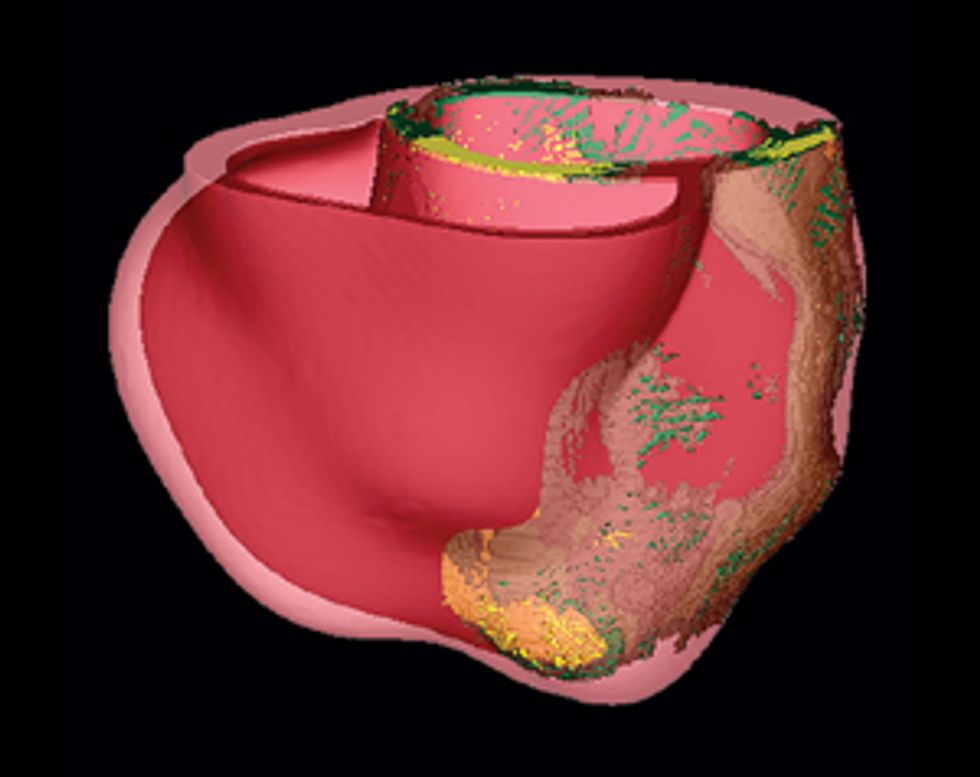
So how do we make a virtual heart? To be clinically useful, our model must represent the individual’s unique anatomy and the pattern of scar tissue from the heart attack. We start with the patient’s magnetic resonance imaging (MRI) or computed tomography (CT) scans, which produce images representing slices of the heart. We use image-processing techniques to identify the muscle tissue in the walls of the heart’s chambers and to map the damaged heart’s scar tissue. Then we use that information to build a geometric model. For the final step we use the images to estimate the orientation of the muscle fibers, which determines how electrical signals propagate through the tissue.
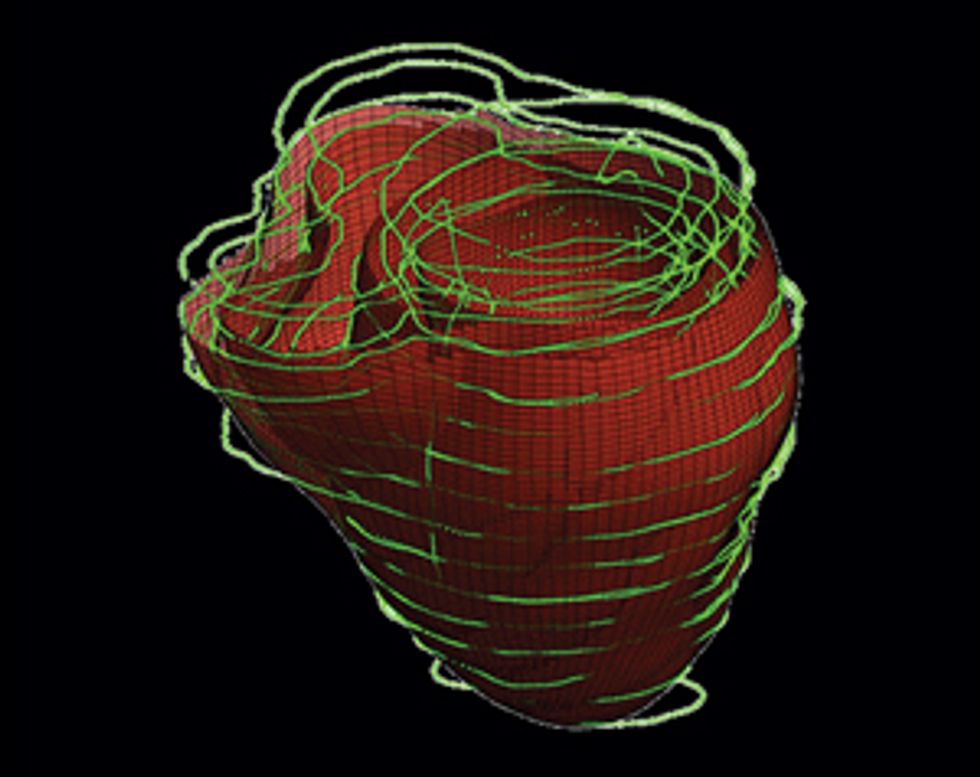
Once we have this patient-specific geometric structure, we overlay a computational model of the inner workings of a generic heart. We need to represent activity at the cellular and molecular levels, where ionic exchanges across cardiac-cell membranes trigger contractions and where currents flow from cell to cell. The result is a personalized computer model that can be likened to Google Earth—think of it as “Google Heart”—which allows us to zoom in on a disease component and then zoom out to explore the phenomena at the organ level.
In another application of virtual-heart technology, we’re looking at a treatment for arrhythmia that goes a step beyond the implanted defibrillator described above. Those devices can lower patients’ risk of dying from sudden cardiac arrest, but they don’t “cure” patients by preventing arrhythmias altogether. So for some patients with dangerous arrhythmias, such as people who receive frequent and painful shocks from their implanted defibrillators, doctors set out to find sources of the problems and fix them.
Let’s imagine that our patient, Jim, is a candidate for this treatment. First, his doctors must study the electrical pulses that are creating his irregular heartbeat. These pulses sweep through Jim’s cardiac muscle in patterns that look like spiraling electrical tornadoes. If the doctors can locate the spot where these disruptions originate, they can burn it away (ablate it, in medical speak) and restore a regular rhythm to Jim’s heart.
Sounds sensible—but the task isn’t easy. To find that critical spot, they bring Jim to an electrophysiology lab and sedate him. Specialists insert a catheter into his leg and slowly guide it through a vein until it enters the heart’s chambers. Then they begin the painstaking process of “interrogating” the heart with the electrode on the catheter’s tip. They slowly navigate the tool around the inner surface of the heart to measure the electric signals in the tissue, trying to piece together a map showing how the irregular electric signal moves through Jim’s heart.
This painstaking procedure typically takes between 4 and 12 hours, and it carries numerous risks, including the possibility that the electrode will pierce the heart wall. The dangers are justified by Jim’s medical condition, but unfortunately this intervention may not help him much. Maps constructed by this point-by-point interrogation method have poor resolution and are sometimes inaccurate. When the doctors use these maps to plan their ablation procedures, they simply don’t have the best information.
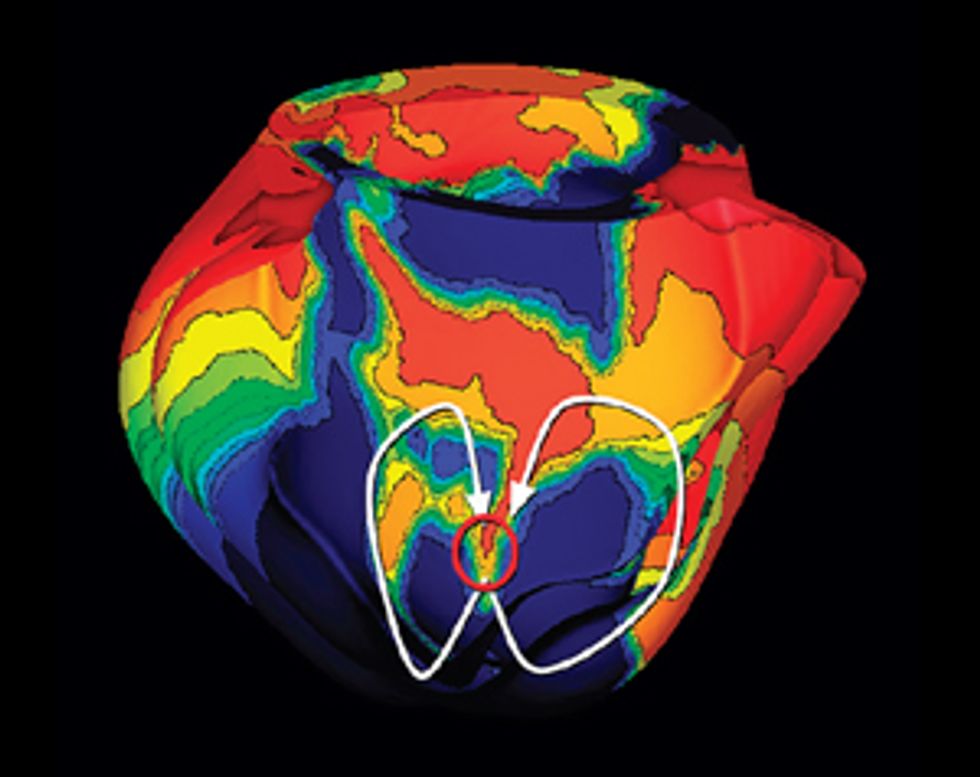
Pinpointing the Problem
Some patients who’ve had heart attacks develop life-threatening arrhythmias, which doctors try to stop by burning away problematic tissue. A computer model can precisely identify the target.
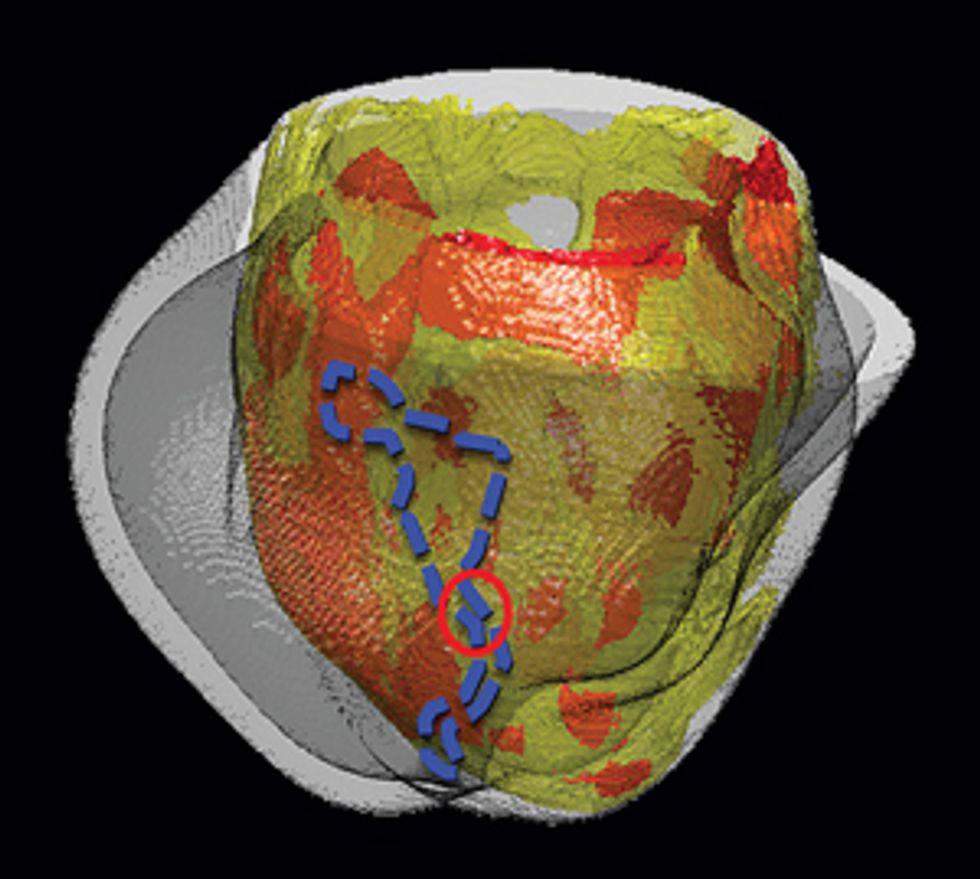
For arrhythmias originating in the heart’s primary pumps—the ventricles—ablation isn’t always successful. Doctors may scorch the wrong tissue, damaging parts of the heart that aren’t responsible for the faulty electric pulse. In one clinical study of patients with an accelerated rhythm called ventricular tachycardia, researchers found that ablation stopped the arrhythmia in only 54 percent of patients, and 8 percent of patients experienced complications.
For people in Jim’s situation, a virtual heart could offer a better way. Indeed, at Johns Hopkins my colleagues and I are now testing the first clinical application of our models in post-heart-attack patients who have developed ventricular tachycardia, which can be life threatening. We want to determine whether our models can help physicians make better decisions about which tissue to burn away—and let them make those decisions without long sessions of probing patients’ hearts.
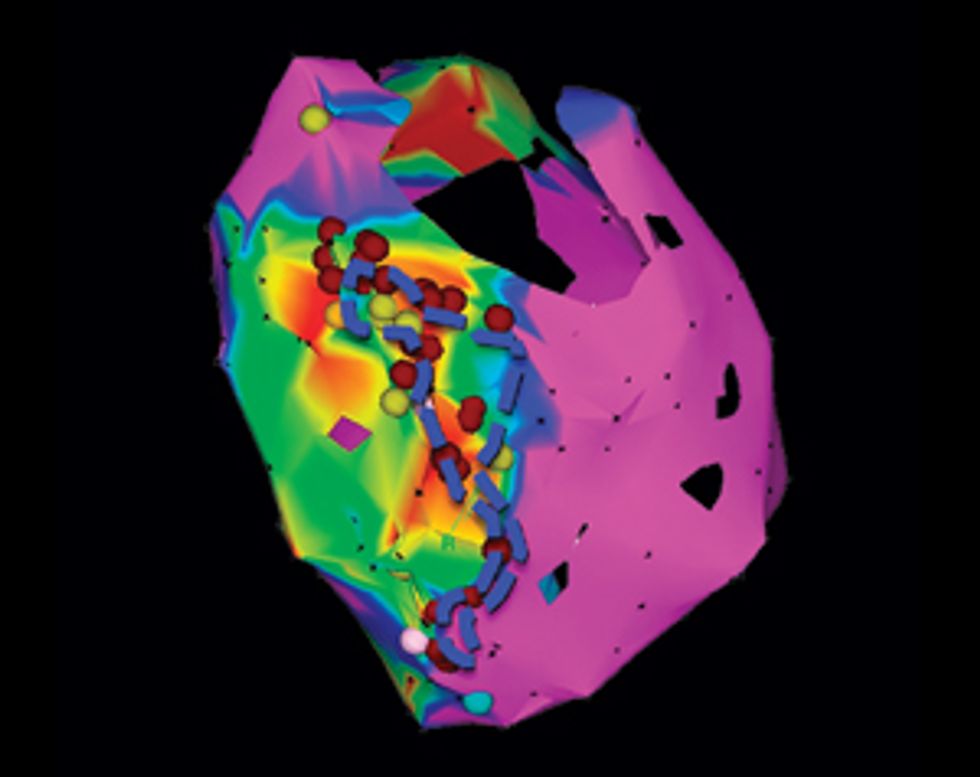
Using our system on Jim, we’d build a three-dimensional model of his heart, which would display its unique structural quirks and the specific patches of dead tissue causing electrical interference. We would then run programs on the model to analyze all possible arrhythmias that could develop in his heart and to locate the tissue responsible for the faulty electric pulses. We could seek ablation targets that provide the most efficient fix, stopping the arrhythmia with the fewest and smallest burns. Then the doctor could navigate the ablation tool to those precise locations in Jim’s heart and destroy the minimum amount of tissue needed to do the job. We believe this would significantly shorten the ablation procedure, decrease complications, and increase the rate of success.
My team has already conducted a retrospective study, in which we used our virtual hearts to study patients who had undergone the traditional treatment. We identified small and precise target spots for ablation, which our simulations showed would eliminate the patients’ arrhythmias. When we compared our targets with the regions that doctors had actually burned away, we found that our targets did indeed fall within those destroyed regions but were much smaller.
In upcoming clinical trials, we’ll identify targets for patients who haven’t yet undergone ablation treatment. The doctors will burn away just our small suggested targets and will then try to induce an arrhythmia with electrical stimulation. If they can’t do it, that will be a clear victory for our method.
Implanted defibrillators have proved their ability to save the lives of patients with arrhythmias, leading cardiologists to authorize their use in an increasingly diverse range of patients. When doctors began implanting defibrillators in children and babies with congenital heart defects, however, they soon ran into difficulties. Here, too, virtual hearts may come in handy.
In a patient with normal heart size and anatomy, the defibrillator is implanted in a standard configuration: A battery sits beneath the collarbone, and a catheter snakes through a vein into the heart’s right ventricle. But existing devices weren’t designed for children’s small bodies, and doctors frequently have to implant the bulky battery down in the child’s abdomen. What’s more, in children with malformed or diminutive hearts, the catheter often can’t get through the tiny veins or reach the proper target inside the ventricle. Surgeons often have to place the electrodes outside the heart instead.
Pediatric hearts with congenital defects are so variable and structurally complex that defibrillator implantation is a highly individualized art. Currently there’s no reliable way to predict the ideal locations for the defibrillator components in a given child, and an imperfect setup can have serious repercussions. For example, poor positioning of the battery can cause the leads that twist through the child’s body to bend and stress, creating fractures in the insulating material. Such cracks can make the defibrillator discharge unnecessarily or, worse, fail to deliver a shock when needed.
The exact location of the electrodes outside the heart is also a question of great importance. Doctors set the defibrillator’s voltage based on how much cardiac tissue the current must flow through and the orientation of the muscle fibers in that tissue. High voltages can damage tissues and can also cause great pain, because the current stimulates nerve fibers. So doctors are motivated to find the locations that deliver an effective shock at the lowest voltage.
In a proof-of-concept study, we took the MRI scans from a pediatric patient with a congenital heart defect, in which the right ventricle was dramatically undersized. We created a 3-D model of the child’s heart and the surrounding torso, and we experimented with different locations for the battery and the electrode tips. Our simulations eventually identified the configuration that should produce an arrhythmia-stopping shock at the minimum voltage. If doctors adopt such models, it may take the guesswork out of device positioning and spare young patients from repeat procedures to reposition their devices.
There may come a time when all patients with heart conditions, from babies to octogenarians, have their virtual hearts tucked into their electronic medical records, which doctors can then use to plan their treatments. I look forward to that day, for I have great hope that these simulated hearts will be able to prevent some real human hearts from breaking.
This article originally appeared in print as “Your Personal Virtual Heart.”
About the Author
Earlier in her career, Natalia A. Trayanova created computer models of the human heart for basic scientific research. Now, as a professor of biomedical engineering at Johns Hopkins’ Institute for Computational Medicine, she’s creating customized models for individual cardiac patients. Doctors are now beginning to run simulations on their patients' virtual hearts to test out treatments. Integrating such computer models into clinical practice could “change the paradigm,” she says.