Spinal Stimulation Gets Paralyzed Patients Moving
Implanted electrodes can reach where the brain cannot

Dustin Shillcox fully embraced the vast landscape of his native Wyoming. He loved snowmobiling, waterskiing, and riding four-wheelers near his hometown of Green River. But on 26 August 2010, when he was 26 years old, that active lifestyle was ripped away. While Shillcox was driving a work van back to the family store, a tire blew out, flipping the vehicle over the median and ejecting Shillcox, who wasn’t wearing a seat belt. He broke his back, sternum, elbow, and four ribs, and his lungs collapsed.

Through his five months of hospitalization, Shillcox’s family remained hopeful. His parents lived out of a camper they’d parked outside the Salt Lake City hospital where he was being treated so they could visit him daily. His sister, Ashley Mullaney, implored friends and family on her blog to pray for a miracle. She delighted in one of her first postaccident communications with her brother: He wrote “beer” on a piece of paper. But as Shillcox’s infections cleared and his bones healed, it became obvious that he was paralyzed from the chest down. He had control of his arms, but his legs were useless.
At first, going out in public in his wheelchair was difficult, Shillcox says, and getting together with friends was awkward. There was always a staircase or a restroom or a vehicle to negotiate, which required a friend to carry him. “They were more than happy to help. The problem was my own self-confidence,” he says.
A few months after being discharged from the hospital, in May 2011, Shillcox saw a news report announcing that researchers had for the first time enabled a paralyzed person to stand on his own. Neuroscientist Susan Harkema at the University of Louisville, in Kentucky, used electrical stimulation to “awaken” the man’s lower spinal cord, and on the first day of the experiments he stood up, able to support all of his weight with just some minor assistance to stay balanced. The stimulation also enabled the subject, 23-year-old Rob Summers, to voluntarily move his legs in other ways. Later, he regained some control of his bladder, bowel, and sexual functions, even when the electrodes were turned off.
The breakthrough, published in The Lancet, shocked doctors who had previously tried electrically stimulating the spinal nerves of experimental animals and people with spinal-cord injuries. In decades of research, they had come nowhere near this level of success. “This had never been shown before—ever,” says Grégoire Courtine, who heads a lab focused on spinal-cord repair at the Swiss Federal Institute of Technology in Lausanne and was not involved with the project. “Rob’s is a pioneer recovery. And what was surprising to me was that his was better than what we’ve seen in rats. It was really exciting for me to see.”
The report brought renewed hope for people living with paralysis. The prognosis is normally grim for someone like Shillcox, who has a “motor complete” spinal-cord injury. That level of damage usually results in a total loss of function below the injury site.
Teams of scientists have been working on transplanting stem cells for neural repair and modifying the spinal cord in other ways to encourage it to grow new neurons, but these long-term approaches remain mostly in the lab. Harkema’s breakthrough, however, produced a real human success story and gives hope to paralyzed people everywhere. It presents a viable means of regaining bowel, bladder, and sexual functions, and maybe—just maybe—points the way toward treatments that could give paralyzed people the ability to walk again.
But Harkema’s first experiment involved only one patient, and many researchers wondered whether the improvement they saw in Summers was an anomaly. “The next big question was, Will you ever see these things in more than one subject?” says neurobiologist V. Reggie Edgerton of the University of California, Los Angeles, a collaborator in the Louisville experiments.
The U.S. Food and Drug Administration (FDA) had given Harkema the go-ahead to try the technique in four more paralyzed people. Shillcox put his name in the pool the night he saw the news report. He was selected, and in July 2012 he packed his wheelchair into his retrofitted Dodge Journey and drove himself from Green River to Louisville to begin 18 months of experiments.
The circuitry of the lower spinal cord is impressively sophisticated. Neuroscientists believe that the brain merely provides high-level commands for major functions, like walking. Then the dense neural bundles in the lower spinal cord take over the details of coordinating the muscles, allowing the brain to focus on other things. That division of labor is what lets you navigate a party and focus on the conversation rather than on your steps. After a spinal-cord injury, damage prevents the high-level signal from the brain from reaching the neurons below. Yet those neural bundles remain intact and are just waiting to receive a signal to start the muscles working. Stimulating the lower spinal cord with electrodes can awaken that circuitry and get it functioning, astonishingly, without instructions from the brain.
It has been known since the mid-1970s that direct stimulation of the spinal cord can actually induce the legs to move as if they were taking steps, without any input from the brain. Edgerton and other researchers have demonstrated the concept definitively in paralyzed cats, rats, and a few humans. But in most of these demonstrations, researchers were blasting a large amount of electrical current into the body to force the muscles to move. “Everyone, including us, was hung up on the idea that you have to stimulate at this high level to induce the movement,” says Edgerton. What they missed was that the stimulation was essentially overwhelming the neurons in the lower spinal cord and was actually interfering with their ability to process sensory information that can help the body move on its own.
The neurons in the spinal cord don’t only receive signals from the brain; they also process sensory feedback from the body as the muscles move and balance shifts. The importance of that sensory feedback gradually emerged with some animal experiments Edgerton reported in Nature Neuroscience in 2009. The study suggested that sensory input could actually control the motor commands produced by the spinal cord.
Harkema, a former student of Edgerton’s, ran with that concept. In her experiments with Summers, she stimulated his spinal cord just enough to wake it up and then let the sensory input do its thing. “It’s like putting a hearing aid on the spinal cord,” says Edgerton. “We’ve changed the physiological properties of the neural network so that now it can ‘hear’ the sensory information much better and can learn what to do with it.”
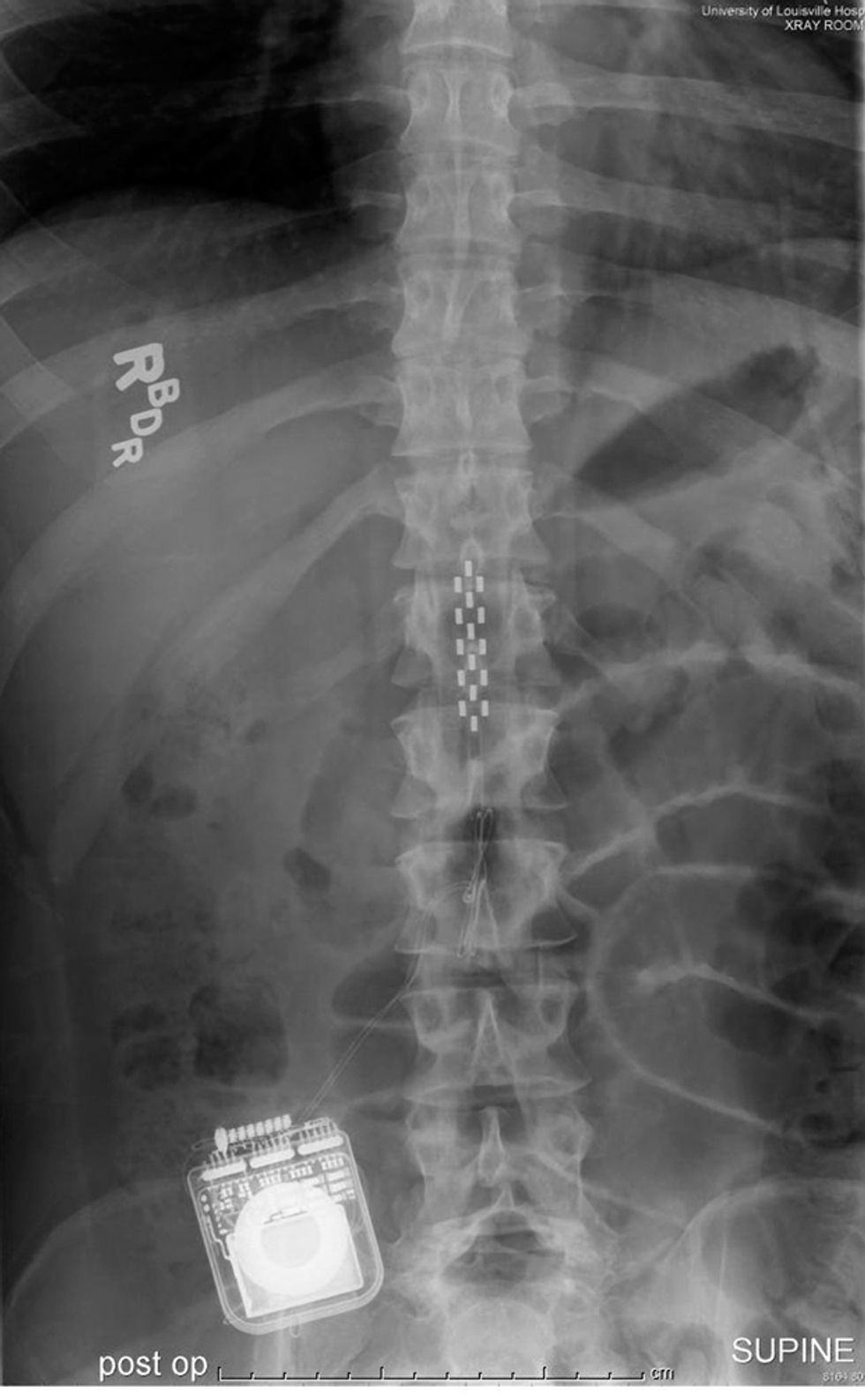
Harkema’s group uses an off-the-shelf neurostimulation system—made by Minneapolis-based Medtronic—that’s FDA approved for pain management. The system’s array of 16 electrodes is surgically implanted in the epidural space next to the outermost protective layer of the spinal cord. The array is then connected to a pulse generator (which resembles a pacemaker) that’s implanted nearby. Finally, the pulse generator receives a wireless signal from a programming device outside the body.
The array spans approximately six spinal-cord segments, the ones generally responsible for movement in the lower half of the body. By placing the electrodes over them, the researchers can generate a response in the corresponding muscle groups. Electrode 5, for example, is located near a segment of the spinal cord that controls hip muscles. Electrode 10 is located at the bottom of the array, over the segment that controls the lower leg.
Each of the array’s 16 electrodes can be set to act as a cathode or an anode or be completely shut off. Stimulation intensities can range from 0 to 10.5 volts with pulses sent at frequencies ranging from 2 to 100 hertz, although the researchers usually don’t go beyond 45 Hz. Picking the right combination of electrodes and stimulation parameters to generate a simple response in a single muscle is relatively straightforward. But generating a complex behavior like standing, which involves many muscle groups and a considerable amount of sensory feedback, is far more difficult. Choosing the right electrode configurations for standing requires both a tremendous amount of intuition and plenty of trial and error. “That’s the challenge: to create the electrical field that’s going to give you the desired behavior,” says Harkema.

On a Wednesday in February of this year, Shillcox arrived at the Frazier Rehab Institute in downtown Louisville for one of his first stimulation sessions. The array and pulse generator had been implanted a few weeks before. He wore Nike sneakers and black gym shorts, revealing thin legs atrophied from lack of use.
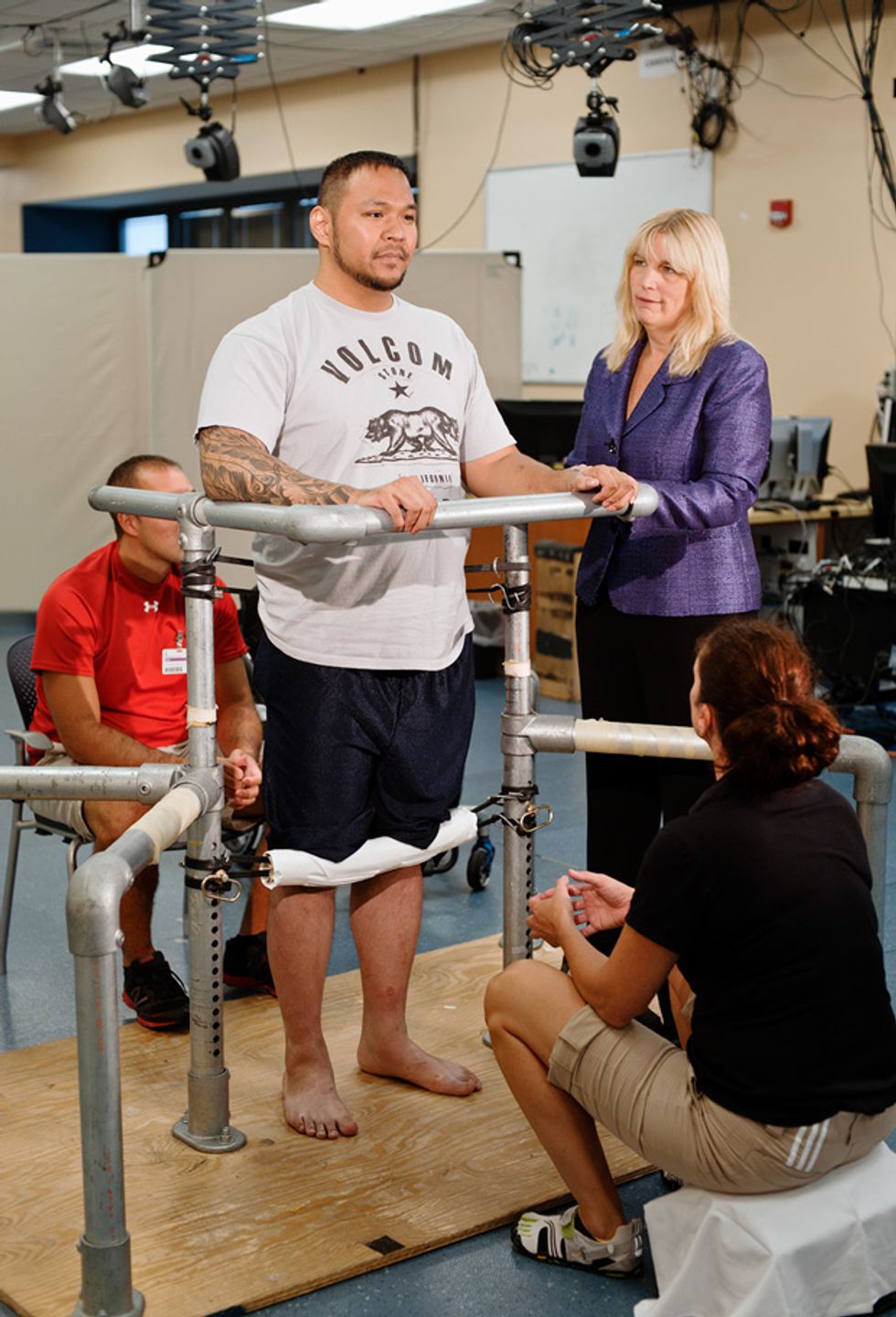
Shillcox joined Harkema and her team in a large room equipped with custom rehabilitation equipment. He wheeled himself to a three-sided stand Harkema had made out of metal pipes that she’d bolted to a piece of plywood. Researchers taped 14 sensors to Shillcox’s legs. Using electromyography (EMG), these sensors would measure the electrical activity produced by his muscles and indicate how Shillcox was responding to the stimulation. Two trainers hoisted Shillcox from his wheelchair onto his feet and into the stand. Then they took their positions to keep him upright—one in front of Shillcox with both hands pushing against his knees and the other behind, steadying his hips. Shillcox held onto the stand with his hands, and a bungee cord supported him from behind.
That day, Harkema planned to test new stimulation configurations to see whether one of them would allow Shillcox to stand on his own. She took a seat in front of a screen displaying the EMG signals while two other researchers helped monitor the data from other screens. To start the session, Harkema called out the electrode settings: “1+, 2+, 3+, 9+, 14+, 12+, 13+, 6+, 7–, 8–, 4–, 10–.” This configuration used 12 of the 16 electrodes, 8 of them as anodes (positively charged) and 4 of them as cathodes (negatively charged). Harkema instructed her team to set the pulsation frequency at 30 Hz and the initial intensity at 1 V and to ramp up by a tenth of a volt at a time. “Left independent,” a trainer called out when the stimulation reached 1.5 V. Shillcox bore his weight on his left leg without assistance for about 30 seconds.
Harkema jotted in her lab book and instructed the team to turn off electrode 10, the one targeting Shillcox’s lower leg. “Going to zero,” a researcher called out. He powered down the system, punched in the new electrode configuration without electrode 10, and ramped it up again. At 2.6 V, Shillcox’s knees buckled. “It shot me out,” Shillcox said. The electrodes hadn’t sent the signal to the legs to stand straight but had twitched his knees forward instead. The stimulation pattern and parameters weren’t quite right.
Harkema tried more configurations, but each time Shillcox felt nothing until Harkema hit a particular voltage threshold, at which point Shillcox’s knees would give way. After 75 minutes, on the 10th and last try, Harkema removed the bungee supporting Shillcox from behind. The muscle activity on the EMG monitors skyrocketed. He’d been balancing so perfectly with the bungee cord that he hadn’t been getting enough external sensory information to activate his muscles, Harkema concluded, so there had been little input flowing back to the lower spinal cord. She instructed her team to devote the next few sessions to the last electrode pattern of the day, but without the bungee.
The technological limitations of the stimulation system make these trials unnecessarily difficult. Each time Harkema changes the configuration of electrodes, she has to turn off the electric field they generate and start over at 0 V. It’s a safety feature of this off-the-shelf stimulator, but it destroys the body’s neural momentum. “You can get really close, and you think the person is almost standing independently, and if you could just shift the field a little you would have it. But you can’t. You have to go to zero. And then everything starts over,” says Harkema. The limitation makes it especially difficult to induce a stepping motion in her patients. “It’s a left-to-right problem. If we get the right leg to step, the left is doing nothing,” she says.
It doesn’t help that there are something like 4.3 x 107 possible electrode patterns she can try and that each can be tried with a range of frequencies and voltages. Without an algorithm to help her choose parameters, Harkema must rely on her experience, some limited neural mapping data, and what she sees on her monitors. “I have to look at the EMG data whizzing by and then make decisions about what I can change out of these 4.3 x 107 combinations to get it better,” says Harkema. She’s gotten pretty good at making adjustments, but she acknowledges that no one can fully interpret the nuances of all that EMG data.
To do better, Harkema has enlisted the help of a handful of engineers who say they can build a stimulation system specifically for her research. At the California Institute of Technology, mechanical engineer Joel Burdick is developing a machine-learning algorithm that aims to take some of the guesswork out of choosing stimulation parameters.
The algorithm is based on statistical methods that predict the patient’s likely response to stimulation patterns—even those that haven’t been tested yet. The prediction part is crucial because there’s no way to try out all the options: There are millions of electrode configurations, and every patient is different. And just to make things even more complicated, patients’ spinal cords change during the course of the stimulation experiments. “The amount of time it would take to test that space is beyond a patient’s lifetime,” says Burdick. So the algorithm has to learn quickly. It must apply reasonable stimulation patterns and then use the patient’s EMG responses to choose better configurations.
Burdick’s team is working with Edgerton’s lab at UCLA to test the algorithm on paralyzed rats. The researchers are starting simply, using just a couple of electrodes and trying to maximize the response in a particular muscle. The first step is to make sure the algorithm is making reasonable decisions. The team has also begun a small human pilot study, Burdick says.
Meanwhile, John Naber, an electrical engineer at the University of Louisville, and a team of engineers are developing a stimulation system that would give Harkema independent control of all 16 electrodes in Medtronic’s array. The design would allow her to transition from one configuration to the next without shutting off the current. The team is building a new pulse generator using off-the-shelf components, and they’ve already written the code and roughed out a design. The challenge, Naber says, will be getting it approved by the FDA in a reasonable amount of time. “It’s not like a commercial integrated circuit or product, because of the FDA requirements for human implants,” Naber says.
The lingering question is whether Medtronic’s 16-electrode array is the best one for Harkema’s work. It was designed to treat pain, so the current diffuses rather broadly. Yu-Chong Tai, an electrical engineer at Caltech, thinks that an array with smaller electrodes arranged more densely might offer the precise stimulation needed after spinal-cord injury. The prototype he’s testing in rats has 27 electrodes arranged over a 2-centimeter-long array. A human version would be similar in size to Medtronic’s (about 5 cm long) but would contain hundreds of electrodes. Of course, more electrodes would mean exponentially more configuration options. “If we give them more electrodes, they will need a smart algorithm,” says Tai.
Until Naber and Tai’s prototypes can be approved by the FDA and Burdick’s algorithm can be fine-tuned, the Medtronic system will have to suffice. That may limit what Harkema can achieve when she puts Shillcox and her other research subjects on the stimulator, especially in terms of stepping. Even so, Shillcox has reason to hope that the experiments will boost his quality of life. Rob Summers, Harkema’s first subject, says his perspective on life has greatly improved since he regained bladder, bowel, and sexual functions. “This project has given me my freedom back,” he says.
Research subjects No. 2 and No. 3 have completed their initial trials. Like Summers, both were able to stand while on the stimulator, as Harkema and her colleagues reported at a Society for Neuroscience meeting in 2012. The researchers have not publicly announced whether other voluntary movement and physiological functions, such as bladder control, have returned for those individuals.
Shillcox—subject No. 4—remains hopeful, but he’s trying to keep his expectations realistic. “I don’t want to be too optimistic, and I’m trying to be prepared for no results at all,” he says. “I hope that whatever they find from this research will at least benefit other people.” Shillcox will likely complete his training by the end of the year, and Harkema says she cannot yet publicly reveal their preliminary results. Whatever the medical benefits ultimately prove to be, working with Harkema as a pioneer on an experimental treatment for spinal-cord injury has boosted Shillcox’s confidence around others. “I have no problem asking for help now,” he says.
This article originally appeared in print as “An Electrifying Awakening.”
About the Author
Emily Waltz, a freelance journalist based in Nashville, frequently writes about biotechnology for IEEE Spectrum. Last year she strapped on an assortment of health-monitoring gadgets for an article about the “quantified self” movement.


