Solid-state lithium-ion batteries promise to be more safe, lightweight, and compact than their conventional counterparts. However, metal spikes can grow inside them, leading to short-circuit breakdowns. Now a new study finds that applying pressure on these batteries may be a simple way to prevent such failures.
Conventional batteries supply electricity via chemical reactions between two electrodes, the anode and cathode, which typically interact through liquid or gel electrolytes. Solid-state batteries instead employ solid electrolytes such as ceramics.
Solid-state batteries can provide more energy than conventional batteries for the same amount of weight or space. Solid-state lithium-ion batteries are also much safer than their conventional counterparts, which use organic liquid electrolytes that are flammable.
“Understanding why and how solid electrolytes fail points us towards new ways to design better solid-state batteries that have higher performance and are more reliable.”
—Cole Fincher, MIT
However, a major problem that solid-state lithium-ion batteries face are branchlike metallic filaments known as dendrites, which can build up on the surface of the solid lithium metal anode. These can penetrate the solid electrolyte and make contact with the other electrode, triggering a short circuit.
For nearly 50 years, scientists have debated over how these dendrites grow. And while it has festered, this mystery has hampered the effort to make solid-state lithium-ion batteries a practical reality.
“Batteries seem like very simple devices, but they are in reality very complicated,” says study senior author Yet-Ming Chiang, a materials scientist and electrochemical engineer at MIT. “And it takes a tremendous amount of research and development to make them work at a practical level.”
Now researchers may have finally answered the question of what makes these dendrites grow. Their new study also reveals a potentially simple way to keep them from short-circuiting batteries.
Scientists had suggested two ways these dendrites might grow. One possibility was a chemical mechanism—the dendrites grew within the electrolyte. The other was mechanical in nature—cracks that formed within the electrolyte let dendrites creep in.
Previously, Chiang and his colleagues unexpectedly found that lithium, which is a very soft metal, could nevertheless penetrate the hard electrolyte used in solid-state lithium-ion batteries. They discovered that as the batteries charged and discharged, the movement of ions caused the electrodes to expand and shrink. This generated stresses in the solid electrolyte sandwiched between the electrodes.
The prior findings suggested these stresses might exert pressure on any microscopic flaws within the electrolytes. This could lead to fractures that allowed dendrites to grow and the electrolyte to undergo mechanical failure. This also suggested that applying pressure on the batteries might spur or suppress dendrite growth.
“The ‘aha’ moment for us was the recognition that if electrochemistry produces mechanical stresses, then the converse must be true—we should be able to use mechanical stress to control electrochemical behavior.”
—Yet-Ming Chiang, MIT
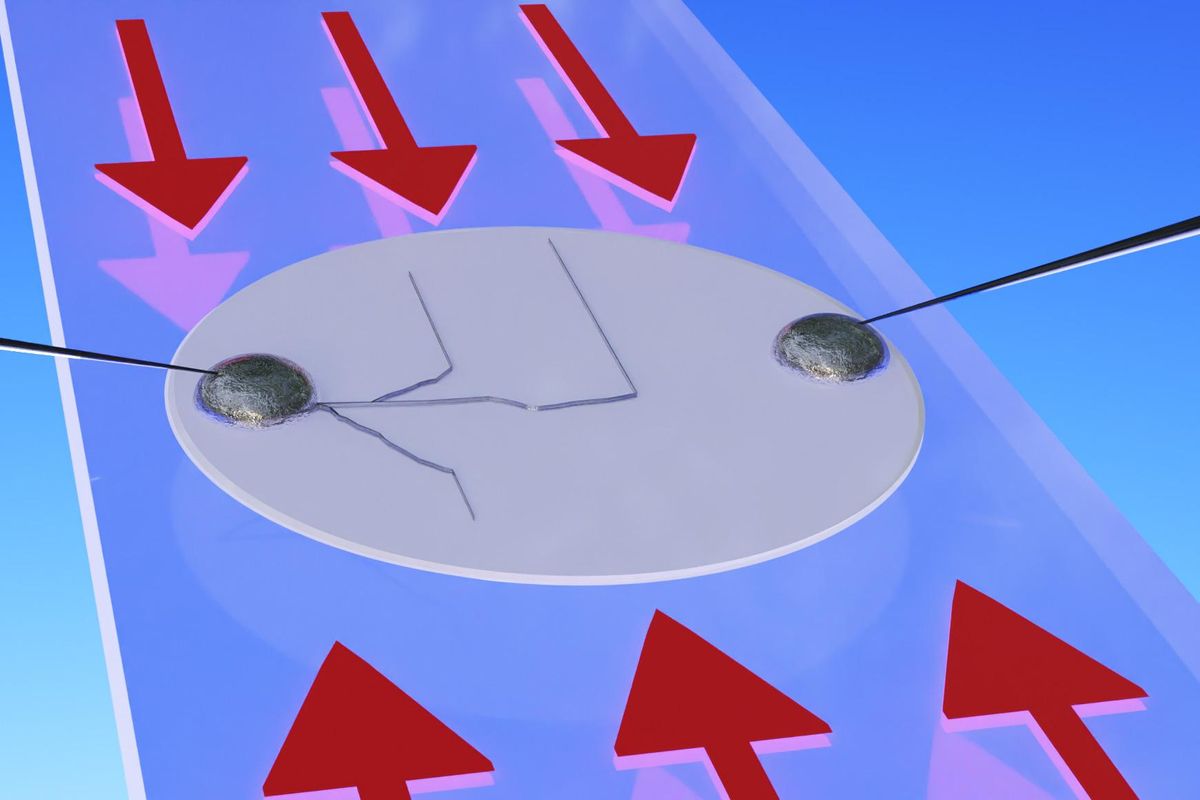
Normally, scientists cannot directly observe dendrite growth in these batteries, as it occurs within the opaque components of the battery cells. In the new study, the researchers developed a way to make thin cells using a transparent electrolyte. This let Chiang and his colleagues see how the dendrites grew and analyze what effects pressure had on them.
The scientists found that applying pressure could deflect the dendrites along the direction of the force. This suggests that mechanical failure is the primary driver of dendrite growth in these batteries, with chemical degradation playing a negligible role.
“This topic falls in a relatively new field of inquiry called ‘electrochemo-mechanics,’ meaning that it is the interaction between electrochemistry and mechanical stress,” Chiang says. “The ‘aha’ moment for us was the recognition that if electrochemistry produces mechanical stresses, then the converse must be true—we should be able to use mechanical stress to control electrochemical behavior.”
Although these new results do not point to a way to eliminate dendrite formation, they do suggest a means to control the direction of their growth. This could lead to strategies to control dendrite trajectory, preventing them from crossing the electrolyte and rendering them harmless.
“We resolved a scientific puzzle that battery researchers have been debating for a number of years,” says study lead author Cole Fincher, a materials scientist and electrochemical engineer at MIT. “Understanding why and how solid electrolytes fail points us towards new ways to design better solid-state batteries that have higher performance and are more reliable.”
In the new study, the researchers bent the electrolyte to apply pressure on it. However, they say there may be many different ways to produce the stress needed to divert the dendrites.
For example, an electrolyte may possess two layers of material that expand and contract different amounts when heated or cooled, as is the case in some thermostats. This would lead to an inherent bend in the electrolyte.
Another strategy is to lace the electrolyte with ingredients that result in a permanently mechanically distorted state. This “doping” method is used to produce the superhard glass used in the screens of smartphones and tablets, the researchers say.
The researchers note that a kind of stress known as stack pressure is often applied to batteries. This applies force onto the battery plates in a way somewhat like compressing a sandwich by putting a weight on top of it. However, they found that stack pressure accelerates dendrite-based battery failure. To prevent dendrite-induced breakdowns, pressure along the plane of the plates is needed, as if a sandwich was squeezed from its sides.
The scientists note that a pressure of about 150 to 200 megapascals was sufficient to stop dendrites from crossing the electrolyte. Although this might seem like a lot—the pressure at the bottom of the Mariana Trench, the deepest part of the ocean, is only 110 MPa—”such stresses appear in many materials and structures we encounter in everyday life,” Fincher says. “It’s present in your tempered-glass car window, your cellphone screen, Corelle plates, and engineering structures such as composites for automotive and aerospace applications.”
The scientists now plan to create functional prototype batteries based on their new findings and figure out what manufacturing processes might be needed to produce such batteries on a large scale. Future research can also investigate whether this effect is seen in all solid electrolytes, Chiang says.
The researchers detailed their findings 18 November in the journal Joule.
- To Make Solid Electrolytes, Start With a Liquid - IEEE Spectrum ›
- This Solid-State Lithium-Ion Battery Recharges Fast, Protects ... ›
- 3D Printed Solid-State Battery Rivals Lithium-Ion - IEEE Spectrum ›
- Solid-State Batteries Rev Up Electric Cars, Boost Grid Storage ›
- Solid-State Battery Has 2x the Energy—and No Anode - IEEE Spectrum ›
Charles Q. Choi is a science reporter who contributes regularly to IEEE Spectrum. He has written for Scientific American, The New York Times, Wired, and Science, among others.