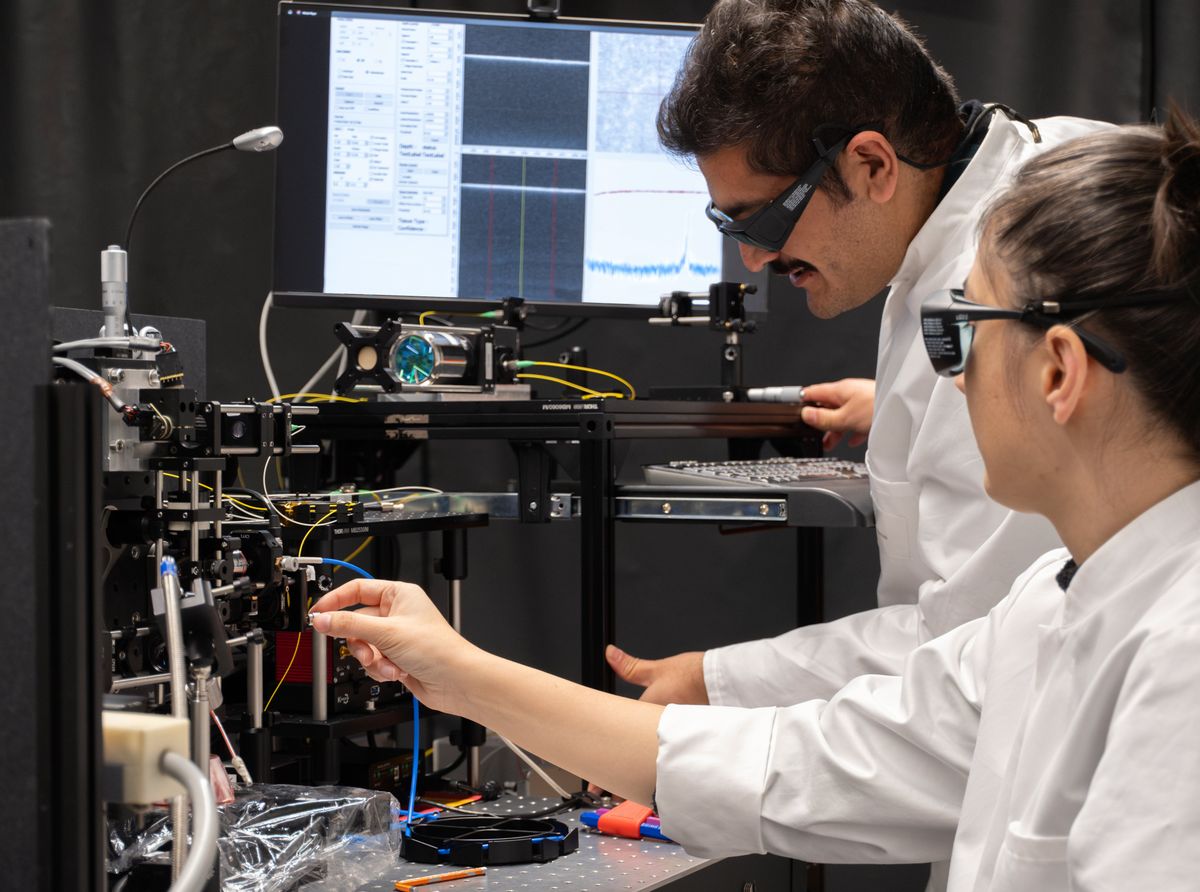
Lasers have been used in medical and surgical procedures for about half a century or so, but scientists are still at working making them safer, faster, and more accurate. Recently, researchers in Switzerland and their collaborators in New York and Dubai have developed a prototype bone-surgery laser system that incorporates real-time visual feedback to monitor and control the depth of cuts and a sensor that differentiates between a patient’s soft and hard tissue.
The system is also autonomous. “There’s no need for external intervention to complete the procedure and protect the soft tissues in the surroundings,” says Ferda Canbaz, a laser physicist at the University of Basel, in Switzerland, one of the lead investigators of the study.
Currently being tested on butchered pigs, the system uses three different lasers—a tissue sensor, a bone cutter, and an optical system. The tissue sensor uses laser‐induced breakdown spectroscopy (LIBS) to create a map of where the soft and hard tissues are. The LIBS system has two components: a series of pulses that are sent to the tissue to be scanned, producing plasma; and a spectrometer that analyses the plasma based on an algorithm. Different tissues have different spectral responses, which allows the sensor to distinguish between hard and soft tissues.
“That helps us to completely protect the soft tissue from being ablated,” says Canbaz. The osteotomy, or bone-cutting, part is activated only when bones are detected on the map generated by the system. This comprises an erbium-doped yttrium aluminum garnet (Er:YAG) laser, which works using a mechanism called photothermal ablation. As the energy of the laser is transferred to the bone tissue, the latter heats up, followed by a pressure buildup, causing micro-explosions that remove tissue.
The third laser monitors the depth of the cut, and makes sure it stays within defined parameters. This is done using optical coherence tomography (OCT), a noninvasive high-resolution 3D imaging method, using light waves to take cross sections of the tissue.
If either the laser or the patient moves for some reason, the laser detects that there is soft tissue in the target region and stops.
All three work in a closed-loop setup, based on a predefined protocol. The laser stops when it reaches soft tissue, and “it also stops when it reaches a predefined depth, if any,” Canbaz says. “We think this could be useful for skull ablation, for instance, when the maximum depth could be predefined. Therefore, even if we don’t detect the soft tissue early enough, we can actually stop the cutting laser.”
Precision is key in laser surgery, and the researchers believe laser-assisted osteotomies (where a bone is cut or lengthened to correct a condition) would allow surgeons to make more accurate cuts. At present, osteotomies are usually done with conventional tools like saws, and lasers, if applied, are used manually or programmed to deliver a certain number of pulses. Surgeons depend on their individual skill and reaction time to stop the cutting or drilling in time to avoid damaging soft tissue. However, an autonomous setup can work without intervention and make decisions about where to cut and where not to.
Canbaz stresses, however, that their tool won’t be a replacement for surgeons, but to make lasers more precise and safer. If either the laser or the patient moves for some reason, she says, the laser detects that there is soft tissue in the target region and stops. A surgical saw or drill causes damage to surrounding tissue, whereas lasers are less invasive because they don’t reach temperatures high enough for killing nearby cells, she adds.
Despite the benefits, there is a lot of work pending before the system can be used for surgeries on humans. One of these is to make it smaller than the matchbox size it currently is. “When you have a miniaturized probe, you have to place it really close to the surface,” says Canbaz. “The debris [left behind from the] cutting process starts accumulating on the surface of your probe and you can’t transmit the laser anymore. So we need to find some solutions.”
The laboratory experiments were done on pig tissue, sourced from the local butcher. When they start working on live tissue, she says, they will need a cooling mechanism. But “when you cool down the tissue, water accumulation gives you the depth results slightly wrong,” she says. Similarly, blood pooling on live tissue also throws off the visualization. This isn’t a safety issue as it results in shallower rather than deeper cuts, but they’d still like to address it.
The researchers are working on expanding this system for other kinds of surgeries as well. They also envisage using it to differentiate healthy tissue from tumors and excise the latter with greater precision. The increased precision of fully automated systems also opens up the possibility of having new cutting shapes. “We want to miniaturize the whole system and make it smart,” Canbaz says. “Which could mean going into the tissue from a small incision, complete the process, and come out.”
- An Ultrasonic Scalpel for Brain Surgery ›
- Today’s Robotic Surgery Turns Surgical Trainees Into Spectators ›
- Google and Johnson & Johnson Conjugate to Create Verb Surgical, Promise Fancy Medical Robots - IEEE Spectrum ›
Payal Dhar (she/they) is a freelance journalist on science, technology, and society. They write about AI, cybersecurity, surveillance, space, online communities, games, and any shiny new technology that catches their eye. You can find and DM Payal on Twitter (@payaldhar).