For the first time, scientists have looked inside a lithium-ion battery as it failed due to overheating. The researchers suggest learning more about such "thermal runaways" can help improve the design and safety of these batteries.
When a lithium-ion battery overheats, it can burn through pockets, burst into flames, or even explode. Although such thermal runaways are rare, they do happen regularly enough to lead some engineers to explore the creation of lithium-ion batteries with their own fire alarms or search for signal processing tricks that can predict fires.
To learn more about how thermal runaway happens—and perhaps how to prevent it—scientists at University College London and their colleagues scanned overheating lithium-ion batteries at the European Synchrotron Radiation Facility in Grenoble, France. The image resolution from the X-rays generated at this particle accelerator is far greater than that of conventional X-ray machines. They detailed their findings online in the 28 April edition of the journal Nature Communications.
Two fully charged commercial lithium-ion batteries were heated to temperatures of more than 250 degrees Celsius using a heat gun. Using X-ray images captured at a rate of 1,250 frames per second, in combination with thermal images, the scientists tracked the rapid breakdown of these batteries’ internal structures before and during thermal runaway.
They found that thermal and electrochemical reactions inside both batteries produced gas pockets that deformed the innards of the batteries. Bursts of heat seen inside the batteries were likely due to internal short circuits when highly conductive parts inside the batteries came into contact.
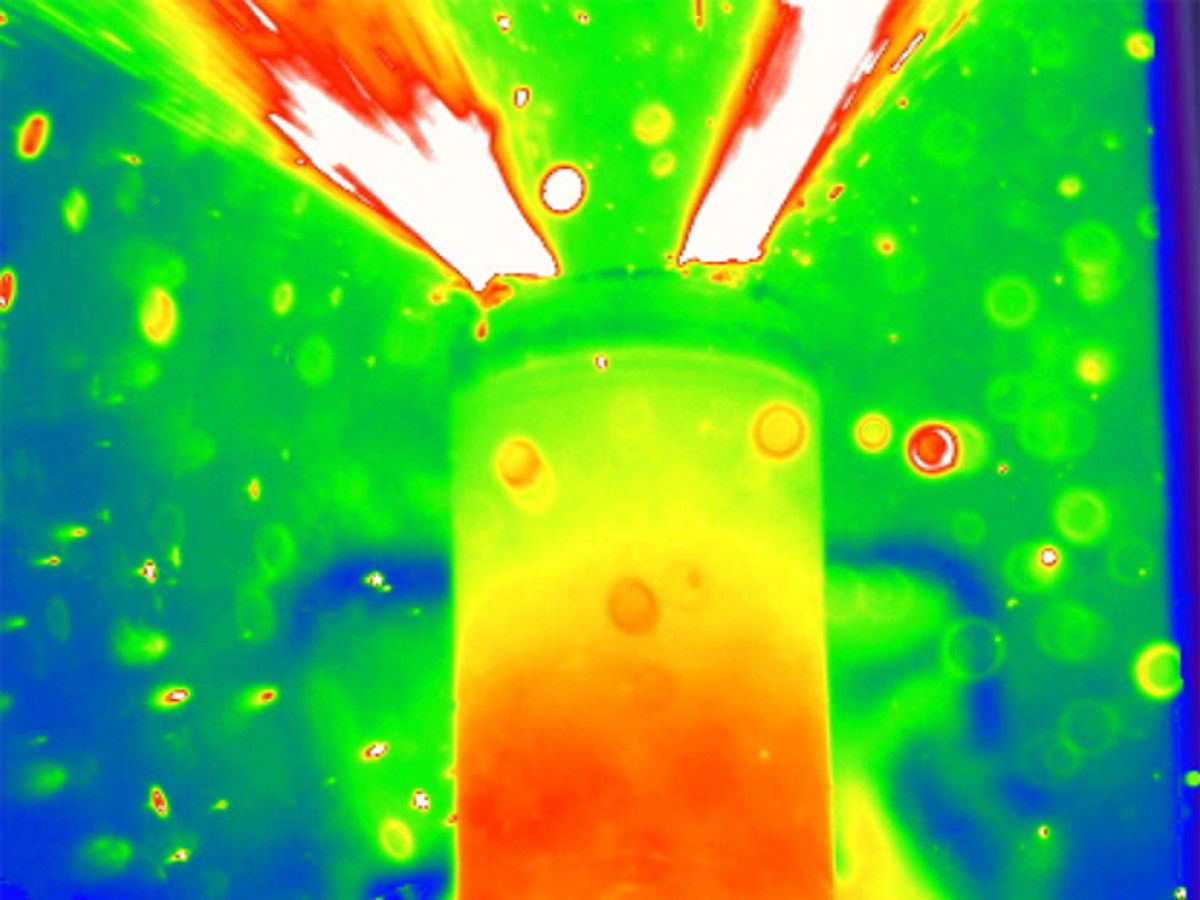
The rapid pressure rise in one battery caused its entire cap to pop off, which in real-world situations would allow oxygen in and increase thermal runaway. In contrast, the other battery's cap remained intact, allowing the heat-generating reactions inside to run to completion, resulting in hot gas and molten material jetting out the battery's vent. Copper inside this latter battery melted, indicating internal temperatures of at least 1,085 degrees Celsius.
The researchers suggest that future analysis can examine catastrophic failures of lithium-ion batteries, which typically result from high rates of recharging and discharging, as well as extreme states of overcharging and undercharging. They added that further studies can also examine the effects of puncturing and crushing.
Charles Q. Choi is a science reporter who contributes regularly to IEEE Spectrum. He has written for Scientific American, The New York Times, Wired, and Science, among others.