The Shocking Truth About Defibrillators
The defibrillators in airports, malls, and offices can save your life—but too many have failed at the crucial moment

In November 2007, Anna Malofiy awoke in her Southampton, Pa., home to find her husband, Eugene, shaking and unresponsive. She called 911 and attempted cardiopulmonary resuscitation. A few minutes later, a police officer arrived with an automated external defibrillator (AED), the Powerheart model, made by Cardiac Science Corp. By administering an electric shock, such devices can save your life if your heart stops beating.
When the officer turned on the device, Anna’s lawyers claim, it displayed an error message and failed to operate. Officers and paramedics attempted to save Eugene Malofiy without the device but were ultimately unsuccessful.
Eugene’s nephew, Francis Malofiy, a lawyer practicing in Philadelphia, is suing Cardiac Science, based in Bothell, Wash., for construction and design defects, a failure to warn consumers about the problems, breach of contracts, and negligence. A jury trial is scheduled for July 2012.
“We went deep into police records and found a smoking gun,” Malofiy says. “Cardiac Science had 114 complaints of these specific relay-switch failures but failed to do any real corrective action. Rather than a recall being issued, rather than anything happening, the situation had to present itself many, many times.”
Cardiac Science did eventually recall about 280 000 of the Powerheart and other models. This is only one of many failures reported of AEDs, first-aid devices that have become increasingly common in the public spaces of the United States, where they are designated by the symbol of a heart and a lightning bolt. There are now 1.5 million AEDs deployed nationwide, five for each of the 300 000 people in the country who need them every year.
When a policeman, shopkeeper, or passerby uses an AED promptly and correctly, it can help keep the suffering person alive until professionals can provide treatment, increasing survival chances up to tenfold. Yet despite the enormous investment in these AEDs, the death rate from sudden cardiac arrest is no better than it was 20 years ago. It still kills more Americans than lung, breast, and prostate cancers and AIDS combined. Worldwide, it kills about 7 million people a year.
So what’s going wrong? Are too many AEDs badly designed or prone to malfunction? Are they just not numerous enough to be found and used in time? Or are there other reasons they aren’t saving lives, reasons that would render public AEDs a waste of money?
First, a primer on the problem. Sudden cardiac arrest is not a heart attack. In a heart attack, blood can’t flow properly to the heart but the muscle itself keeps beating, so sufferers typically remain conscious. In cardiac arrest, the heart’s pumping mechanism—an electrochemically choreographed affair—becomes deranged, so that the many motions of the various parts no longer work together to pump any blood. With no blood flowing to the lungs or brain, victims rapidly lose consciousness.
From that moment on, time is of the essence. For every minute that passes without a heartbeat, the patient’s chance of survival drops by up to 10 percent. Even if a properly trained bystander immediately starts cardiopulmonary resuscitation (CPR), rapidly compressing the patient’s chest to force blood around the body, survival rates will still decline 5 percent per minute. To actually save the person, you must restore the heart’s normal sinus rhythm, and this is where AEDs come in.
Sudden cardiac arrest is most often caused by ventricular fibrillation, when the heart’s lower chambers stop beating and instead quiver rapidly and irregularly. AEDs detect this distinctive quivering and then deliver one or more electric shocks. The shocks cause the heart’s muscle cells to contract simultaneously, interrupting the disorganized spasms and, if all goes well, rebooting the malfunctioning organ.
The first successful use of defibrillation on a person was in 1947, by Dr. Claude Beck of Western Reserve University School of Medicine, in Cleveland. His experimental device required a large transformer and delivered AC current directly to the exposed heart of a 14-year old boy, who was undergoing surgery when he suffered cardiac arrest. The boy survived. Closed chest systems followed in the mid-1950s. Eventually, defibrillators moved to DC current supplied by banks of capacitors, so that defibrillators could be made portable and even battery powered.
Until the 1990s, AEDs delivered what are termed monophasic shocks—jolts so energetic they could damage heart tissue and inflict burns. Modern AEDs take a leaf out of the alternating-current playbook by employing a biphasic waveform: The pulse travels in alternate directions between the paddles, allowing for lower energy levels and providing higher survival rates. In hospitals, biphasic defibrillators can now restore a regular pulse in up to 99 percent of patients.
Today, however, the majority of these machines are situated not in hospitals but in public places, which is pretty much without parallel among medical devices. A public-access AED spends endless days, months, and even years hanging on a wall, gathering dust. Most AEDs reach the end of their expected 5- to 10-year lifetimes without ever having been used. Yet they must remain fully charged, functional, and ready to operate at a moment’s notice, often by people who have never seen a defibrillator outside of a TV hospital drama.
“It was a really daunting technical challenge,” says Carl Morgan, who developed some of the first public AEDs at Heartstream (now part of Philips). “We helped pioneer the concept of self-test, where the device wakes up every day and performs extremely comprehensive tests on itself. These are supplemented by other tests done weekly and monthly to conserve the battery.”
In modern AEDs, he adds, twice as much software code is dedicated to self-testing as to signal processing (for analyzing heart rhythms), and the bulk of the device’s battery power is also reserved for tests. Even so, things occasionally go wrong.
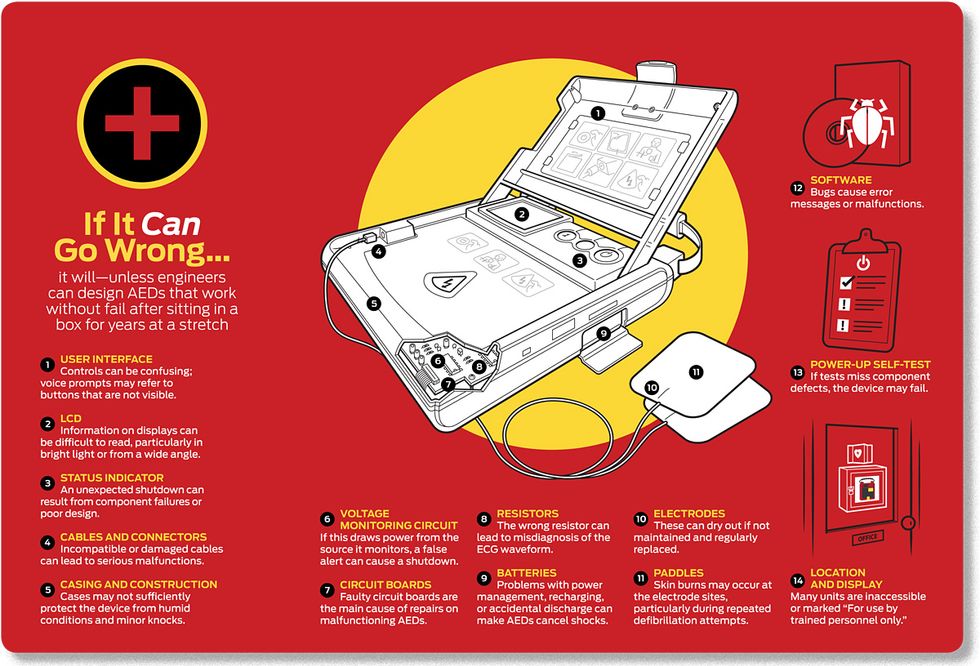
Last year, officials at the U.S. Food and Drug Administration (FDA) noticed a disturbing trend. Between 2005 and 2009, the annual number of problems reported with AEDs increased by 85 percent. Medical-device reports are issued by manufacturers whenever they think one of their units may have malfunctioned, contributing to a death or serious injury. In that five-year period there were more than 28 000 such reports—one malfunction for every 50 devices in the country. More than 750 of the reports followed a death. Problems included the AED displaying error messages, being unable to power up, and failing to deliver shocks. In the same period, up to 70 kinds of AEDs were recalled, including models from every AED manufacturer in the world. Of those recalls, 17 fell into the most serious category, in which there was a reasonable probability that the problem would have led to serious injury or death.
“This opened our eyes,” says Mitchell Shein, who oversees the regulation of pacemakers, defibrillation devices, and the electrical leads attached to both for the FDA. “Was the approval process providing us with adequate control so that devices making it to market were safe and effective? We needed to make an assessment.”
The FDA discovered that manufacturers had evaluated barely a third of the malfunctions and had identified the problem in only a third of those cases. Some 90 percent of the failures were thus unexplained. Worse, because the manufacturers had declined to reveal their sales volume to the FDA, the agency couldn’t nail down the rate of malfunction or tell whether it had risen over time.
“I was stunned to hear this,” says E. Magnus Ohman, a physician on the FDA’s oversight panel for AEDs. “We’re in the era of transparency. If I go to a restaurant, I get the hygiene rating.” Another physician on the panel, Frank LoGerfo, complains, “If these devices can self-report after an incident, it’s almost inexplicable that we don’t have very detailed data for each device where it failed.”
Eventually, the agency set its own engineers to work on the job. “We found some pretty egregious design errors,” says Al Taylor of the FDA’s Office of Science and Engineering Laboratories. “There were many situations where deficient product design and—let’s put it bluntly—poor engineering were a cause or a contributory factor to an adverse event.”
One AED, the brand name of which the FDA would not disclose, was found to occasionally misdiagnose the heart’s electrical rhythm. It delivered some shocks that weren’t needed and failed to deliver others that were. The culprit was a resistor that could vary in resistance by up to 10 percent of its stated value. “When our engineer looked at this design, it was an instant ‘uh‑oh,’ ” says Taylor. “We would have to do analysis to decide if it needed a 1 percent or a 0.1 percent resistor, but this was clearly off the scale.” The reason? Only at that higher tolerance can the noise-canceling circuitry isolate the cardiac signal from environmental sources of radiation, such as the oscillating electric fields of fluorescent tubes.
In another device, a voltage-monitoring circuit drew power from the very source that it was checking. Because of this, a momentary drop in voltage would cause a false signal to shut down the AED, and the device would be unable to deliver a shock. Still another recalled AED—made by MRL, of Buffalo Grove, Ill.—would work perfectly until somebody dropped it. The impact could cause a circuit board connector to perforate an insulation shield, creating a short circuit and generating RF interference that would prevent analysis of the patient’s arrhythmia. Again, the unit would not deliver a shock.
The companies put some of the blame on a manufacturing environment they say has become increasingly globalized in the past 15 years. “Most of the risk today is in the supplied material, the commercial off-the-shelf components; these are the components that go in your iPhones and your laptops, and we don’t control their specification,” says Brian Webster, president of Physio-Control, a division of Medtronic. “We’re prisoners of that supply chain.”
Beyond failures in design and purchasing control, the FDA found that many AED manufacturers were practicing a “fix on fail” philosophy. William Maisel, deputy director for science at the FDA, explains: “Fix on fail means identifying and trending problems on a case-by-case basis, repairing individual devices rather than communicating the problem to all users. In one example, a firm tracked hundreds of complaints tied to a known defect. They serviced each device when it failed but did not systemically notify other users so that their devices could proactively be evaluated and repaired.”
How did the AED industry get into this state? It has to do with a quirk in the law regulating medical devices. If you count AEDs as Class III devices—those intended to support and sustain life—then manufacturers must produce extensive efficacy, safety, and reliability data, usually provided by large-scale clinical trials. This process can cost upward of US $800 000 and take two years.
Manufacturers can, however, get around those requirements, thanks to what’s called the 510(k) process, which effectively removes AEDs from Class III. The process requires merely that a new AED be “substantially equivalent” to any AED on the market. The 510(k) system was originally intended as a temporary measure to grandfather in devices already on the market in 1976. More than 30 years later, the process is still being used to clear AEDs, as well as 25 other high-risk products, including implantable pacemakers, ventricular bypass devices, and systems for electroconvulsive therapy. Meanwhile, external defibrillators have graduated from manual operation that requires some degree of skill to complete automation. They have also been upgraded to administer a biphasic shock, use rechargeable batteries, and incorporate LCD screens, CPR pacing systems, voice prompts, and self-testing circuits. These features add to the things that can go wrong—and as they were introduced, they were grandfathered in.
In a recent report, the Institute of Medicine of the National Academies noted that “Prior 510(k) clearances are legally binding on the FDA when making clearance decisions. Thus, any unsafe or ineffective devices are embedded in the system.” Dr. Michael Carome, a physician with the consumer advocacy group Public Citizen, goes further: “The entire category of AEDs has been insufficiently regulated by the FDA. The status quo essentially represents ongoing, uncontrolled human experimentation with no ethical oversight,” he says.
The FDA is now considering whether to remove the 510(k) loophole for AEDs and classify them as full Class III products. Despite claims from manufacturers that reclassification will double or triple the development time for new devices, the change seems likely to proceed.
Not everyone favors reclassification. “AEDs are fine, technologically,” says Gust Bardy, a physician who helped to found the Seattle Institute for Cardiac Research (and is a consultant for Cardiac Science). “You can always find engineering problems, but patients in cardiac arrest are predominantly going to die. There seems to be an unrealistically high expectation that these machines can reverse the process of death. The more restrictions one puts on AEDs and the more demands for AED perfection, the fewer lives will actually be saved. Innovation will be pushed overseas, and we’ll be stuck with AEDs that are older and more expensive.”
The FDA’s Shein agrees that the vast majority of today’s AEDs are effective. “There is room for improvement, but it would be a poor choice to leave an AED hanging on the wall rather than applying the therapy to a patient,” he says. Rare failures, like that of the unit used on Eugene Malofiy, are of course distressing. But a much bigger factor in the poor survival rate from sudden cardiac arrest is the difficulty of getting access to an AED in the first place. In the United States, AEDs are used in just 4 percent of sudden cardiac arrests that occur in public places. One reason is that AEDs are not always easily located; more on this later. Another deterrent is that many AEDs are labeled “For use by trained personnel only.” These labels arose because virtually all AEDs are prescription-only devices, bought under the authority—and responsibility—of a medical officer at the airport, mall, or school. If an AED were somehow misused—or even correctly used by someone not explicitly authorized by the prescribing physician—that doctor could be held legally liable and theoretically even lose his or her medical license.
In fact, modern AEDs are safe and simple enough for anyone to operate: A 1999 study found that even untrained sixth graders could use them nearly as effectively as trained paramedics. Also, Good Samaritan laws cover the well-intentioned use of public AEDs by lay people nationwide. But discouraging “trained use” labels persist—even for public AEDs found within buildings of the FDA itself.
Worse still are stories of bystanders vainly seeking AEDs that were locked away in offices or languishing in unmarked closets. The solution appears to be education and openness. In 1998, Washington state began a citizen defibrillation program that included advertising, public training sessions, and the creation of a detailed registry of AED locations around the state. The survival rate for witnessed sudden cardiac arrest in Seattle is now more than 45 percent, compared with less than 0.5 percent in Detroit and 4 percent nationwide.
If regional health authorities have much to learn from Seattle, manufacturers could do with some inspiration, too. Critics complain that AEDs lack the plug-and-play simplicity of today’s consumer electronics. “Each manufacturer has different electrodes that attach to the AED differently,” says Robyn Silverman of the ECRI Institute, a nonprofit organization that researches medical technologies. “When the next level of care arrives, they might have another brand of AED. Paramedics have to rip off the first electrodes and put on new ones, which takes time. It would be nice to have universal electrodes for any AED.”
Then there’s the AEDs’ price: up to $2000. “If companies were able to produce low-cost AEDs that could be purchased by nearly everybody, then you’d have mass dissemination, like cellphones,” says Bardy. “But under the current regulatory, legal, and engineering burdens, it’s nearly impossible to produce an AED that can be bought at Costco.”
Ideas abound. BT Group (formerly British Telecom, the United Kingdom’s communications leader), has replaced obsolete wired telephones in some of its iconic red phone boxes with public AEDs. And the San Ramon Valley Fire Protection District, in California, has released one of the world’s first bystander CPR apps, for the iPhone. Users of the app receive an alert when anyone within 500 feet reports a sudden cardiac arrest, along with a digital map pinpointing the location of both the victim and the nearest public AED.
Future AEDs might be integrated into a program called Next-Generation 9-1-1, a federal initiative to expand emergency communications to include wireless and voice-over-IP devices, text and video messaging, and geolocation data. Tomorrow’s AED could automatically summon paramedics the moment it’s activated.
But the companies seem reluctant to invest the necessary money. Says Brian Webster, of the AED maker Physio-Control: “The public access market is only about 15 percent penetrated. The economics of the market today do not support our industry doing core technology innovation.”
Clearly, the AED industry is not in the best of health. It’s ready for a good shock to the system. There are two on the way: reclassification by the FDA and a potentially industry-shifting lawsuit. Whether these jolts will prove fatal or therapeutic remains to be seen.
This article originally appeared in print as “A Shocking Truth.”
About the Author
Mark Harris, a British writer based in Seattle, got the idea for this article when he learned that one in 50 public-access defibrillators have malfunctioned within the past five years. “If 2 percent of iPhones had malfunctioned, it would be headline news,” he says, “and yet these lifesaving devices are far more important.” Harris wrote our August 2010 cover story about MRI lie detection. He has also covered sleep technology for The Sunday Times and open-source surgical robots for The Economist.