How a Microscopic Supercapacitor Will Supercharge Mobile Electronics
Laser-etched graphene brings Moore's Law to energy storage

Capacitors. Open up your computer and they stick out like rocks on a sandy beach. They’re the one kind of electronic device that never made it to Lilliput. If they finally obeyed Moore’s Law by squeezing themselves down to the microscale, it would make life a lot easier for electronics engineers.
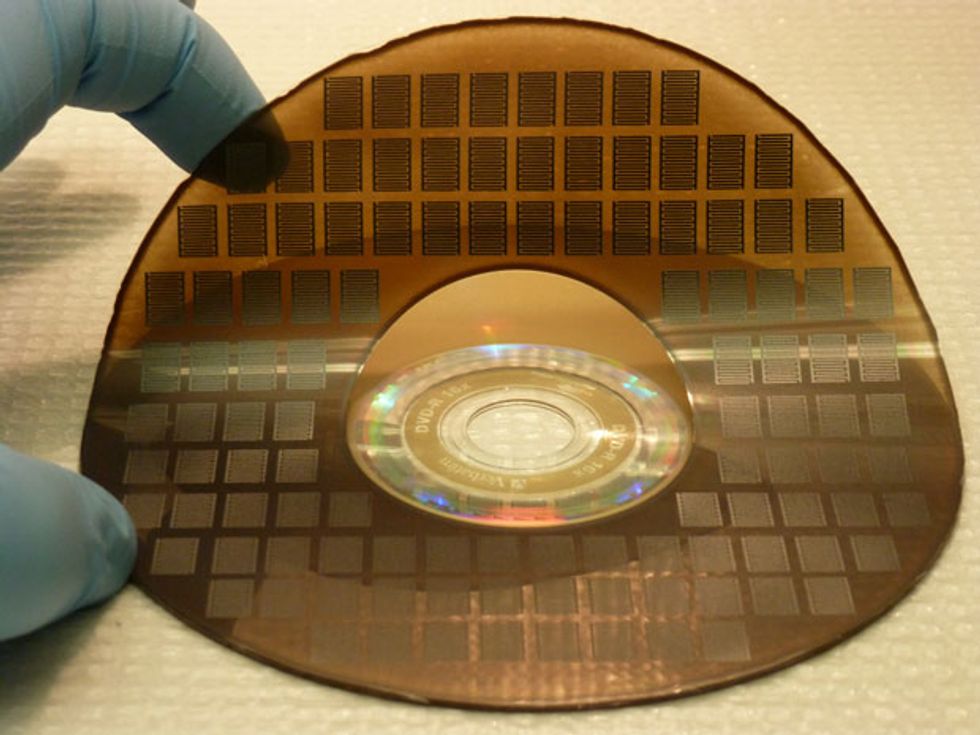
Tiling a Disc With Scores of Super-Capacitors
The authors produce many supercapacitors at a time with nothing fancier than a LightScribe laser etcher, available for the price of a good men’s blazer. Each device consists of graphene electrodes separated by graphite oxide dielectric, topped with a drop of electrolyte.
With tiny but powerful capacitors you could make cheaper, even tinier cardiac pacemakers and computers. They’d be great in nonvolatile memory, microsensors and actuators, RFID tags, and microelectromechanical systems, applications in which the power supplies can weigh up to 10 times as much as the other parts combined. And because, like all capacitors, such devices would be able to release their charge very rapidly, they could be coupled with high-energy batteries to provide periodic surges, as conventional capacitors do to power the flash in smartphone cameras. (Miniaturized supercapacitors could thus lead to even thinner smartphones.)
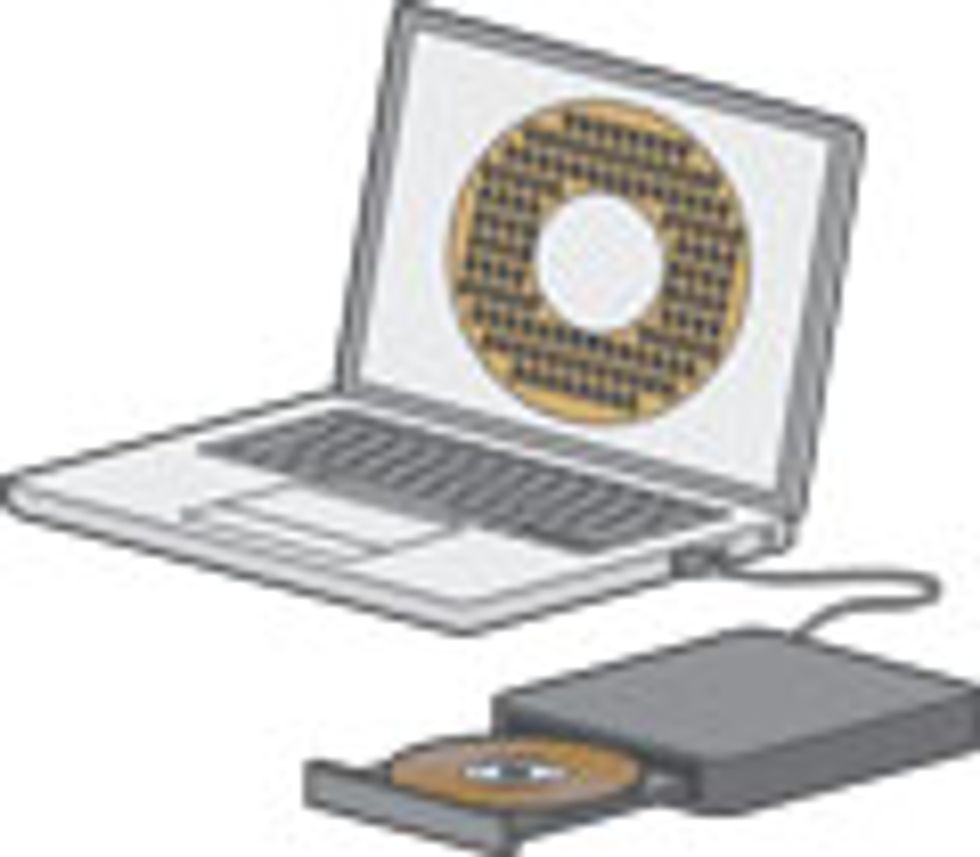
Our group at the University of California, Los Angeles, has created such microsupercapacitors using a simple DVD burner to forge the one-atom- thick sheets known as graphene on which these devices are formed, in arrays. Together with a battery, such supercapacitors could run a cellphone for days. And because an array is less than 10 micrometers thick—far finer than a human hair—it is completely flexible. Build these arrays on flexible substrates and they could power a roll-up display.
All these things can be done at low cost. Our fabrication method can easily be scaled up, and our microsupercapacitors can be readily integrated onto silicon chips. In many cases they can make up for the inherent weaknesses of batteries, such as relatively slow power delivery and long recharge times. So even in those applications where these devices cannot replace batteries, they will augment them enormously.
Consider the lead-acid battery. If Moore’s Law had applied to it for as long as the law has applied to semiconductors, a fully functional car battery would now be the size of a red blood cell. But this technology, invented in 1859, matured long ago. Nickel-cadmium and nickel-metal-hydride battery technology matured more recently, but they, too, are close to their theoretical limits for power and energy density. Even lithium-ion batteries—which have tripled in energy density since the early 1990s—are near the end of their technological growth spurt.
Batteries can’t follow Moore’s Law because no known material can pack an immense charge into a small volume. And the microbatteries that we do have are expensive because they are made using complicated and time-consuming processes. Nor are we likely to find salvation in the various energy-harvesting schemes that have been suggested; they just don’t give product designers the necessary performance and reliability.
But capacitors offer another form of storage. And we have found a way for them to hitch a ride on Moore’s Law.
A traditional capacitor is made of two metal plates separated by a thin insulating layer. It stores charge electrostatically, in an electric field created by the two oppositely charged plates. How much charge can be stored is determined by the capacitance of the device. It is a function of the area of one of the metal plates—typically less than a square meter—divided by the spacing between them, which is typically about a micrometer or less. Therefore, to increase the charge you must maximize the area and minimize the distance.
Supercapacitors minimize the distance by borrowing a bit of battery technology—the electrolyte. A supercapacitor is defined as an electric double-layer capacitor. It consists of two electrodes impregnated with a liquid electrolyte and separated by an ion-permeable layer to prevent short circuits between the electrodes. As voltage is applied, ions from the electrolyte move onto the surfaces of the electrode of the opposite charge. Charge accumulates at the interface between the electrodes and the electrolyte, forming two charged layers, or electric double layers, that are separated by only a nanometer or so.
Our form of supercapacitor also improves area, the other variable. Graphene’s atom-thick sheet has the highest possible ratio of surface to volume: A gram of it could pave 2,630 square meters—nearly two-thirds of an acre. Together with the 1-nanometer separation of charge, this yields a capacitance a million times that of a garden-variety ceramic capacitor and about 10,000 times that of a typical electrolytic capacitor.
Our interest in energy storage goes back to the early 1980s in Neil Bartlett’s lab at the University of California, Berkeley, where one of us (Kaner) was a postdoctoral scholar, working on new forms of graphite. This type of carbon is widely used in lithium-ion batteries today because it is inexpensive, highly conductive, and able to store lithium ions efficiently. However, its low surface-area-to-mass ratio limits its ability to store charge. But it gave Bartlett an idea: to fabricate a “holey” graphite with the surface area needed to store plenty of charge. Unfortunately, his Berkeley team couldn’t then realize this top-down idea of drilling 3-D holes at the atomic scale.

It took 30 years, but we finally did solve this problem in our lab at UCLA, with a bottom-up approach. We started from individual sheets of graphite oxide—a 150-year-old material that is hydrophilic and therefore easily processed in water into films or coated onto virtually any substrate. A few years ago, we found that by shining intense laser light on graphite oxide we could boil off the oxygen in the form of carbon dioxide, leaving behind a 3-D form of graphene—holey graphite. Essentially, we are assembling 2-D graphene sheets into a macroscopic 3-D network that looks like corrugated cardboard.
This corrugated carbon has extraordinary properties. We found that electrons moved 100 times as fast in our 3-D graphene as in the graphite used in batteries and 10 times as fast as in state-of-the-art carbon nanotubes. That speediness meant that supercapacitors could be an excellent application.
We had one more technical problem to overcome: geometry. Traditional supercapacitors consist of pairs of electrodes stacked vertically, like the stories of a high-rise building, but most integrated systems are laid out flat and thus can accommodate only single-story structures. We needed a planar geometry that could be fabricated in a way that would be compatible with modern microelectronics.

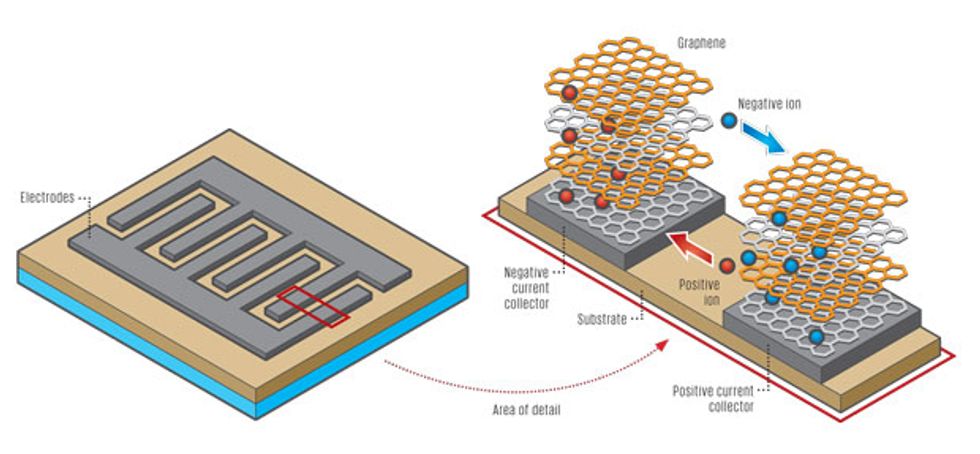
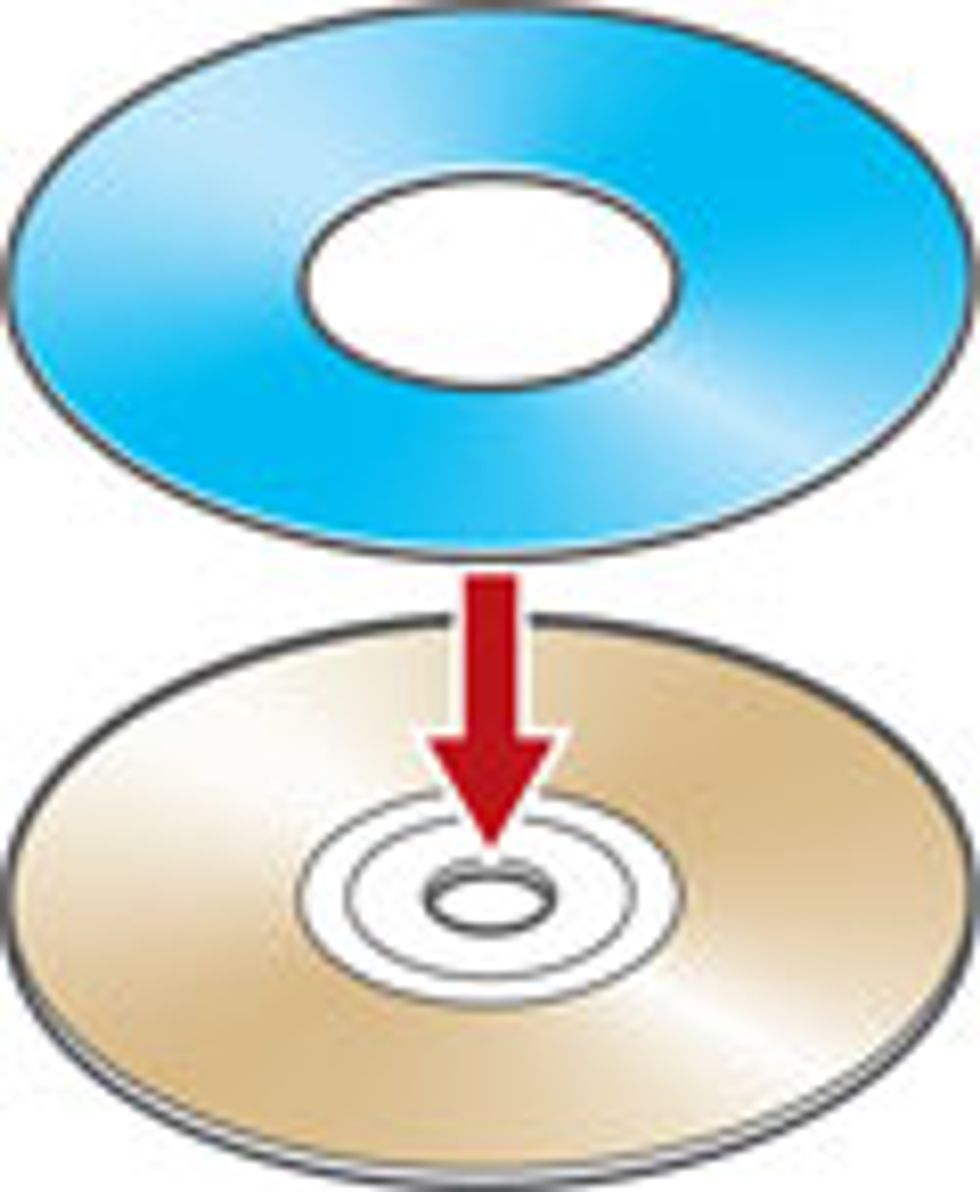
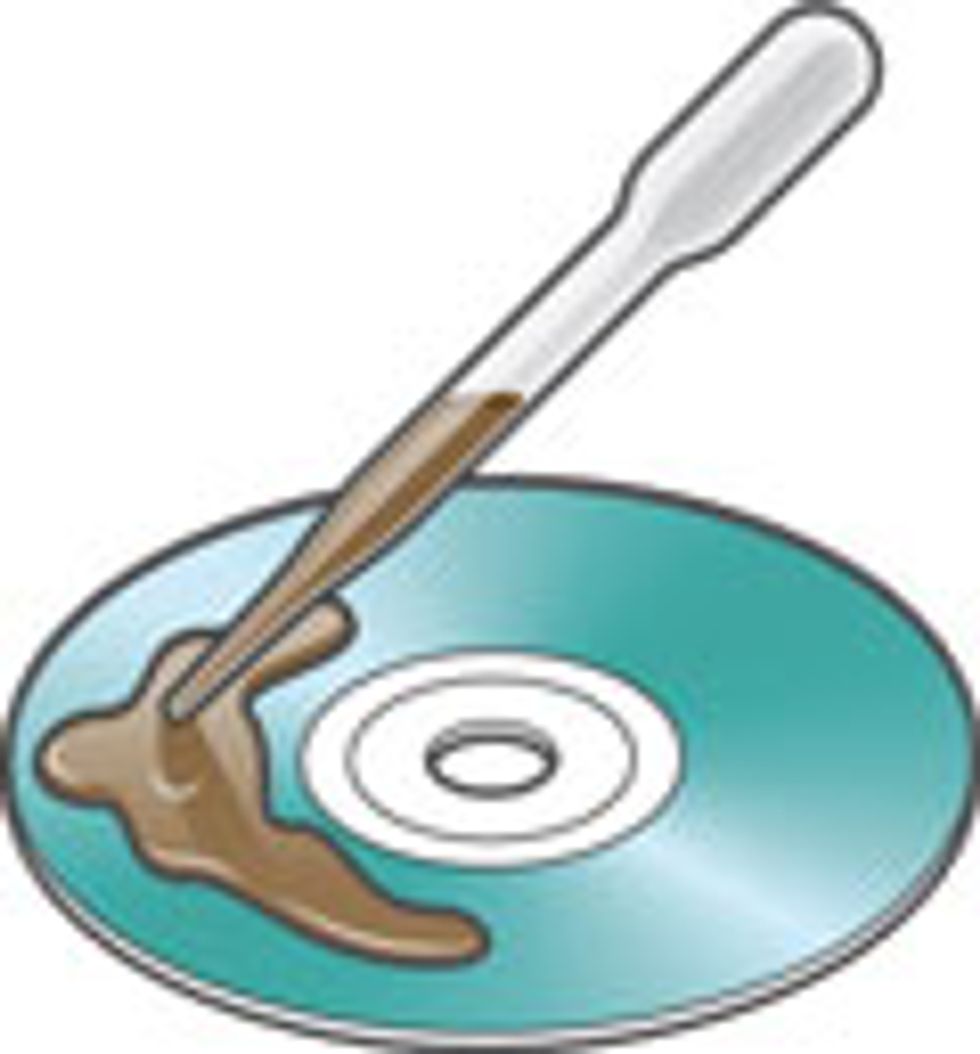
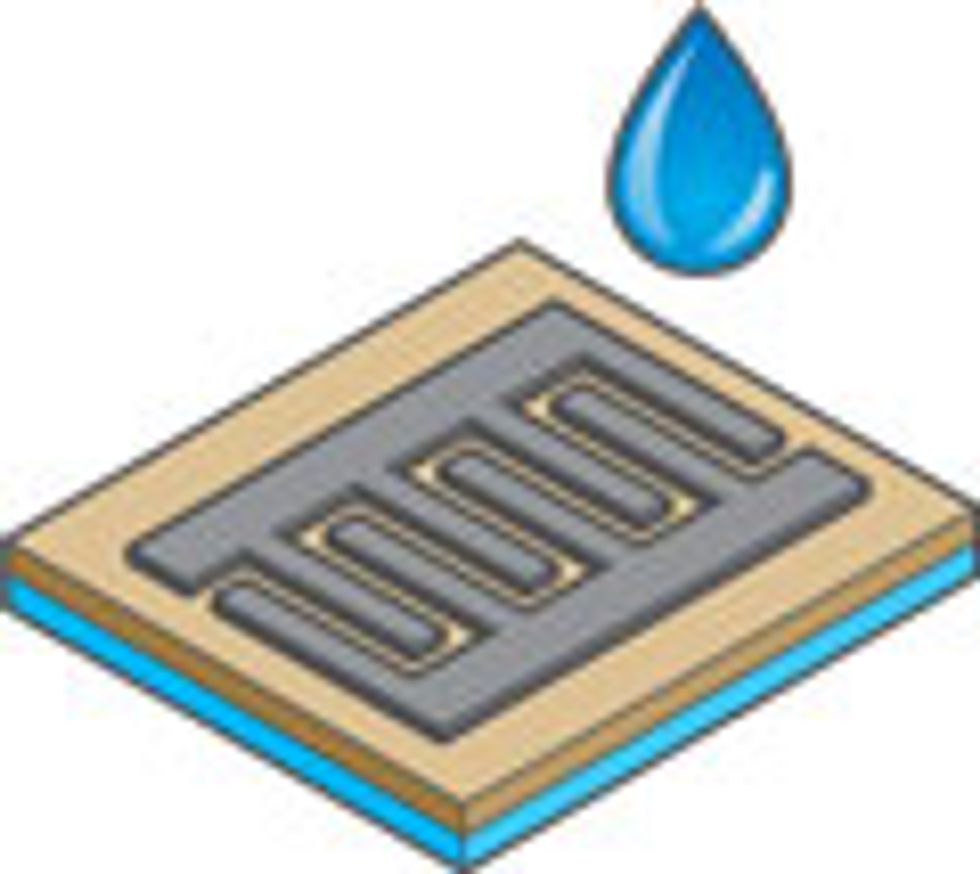
We found the solution in LightScribe, an inexpensive, off-the-shelf laser technology that millions of people have used to etch labels and designs onto compact discs. However, instead of using a disc coated with a reactive dye that changes color on exposure to laser light, we use a very thin coat of graphite oxide. The laser heats the oxide, transforming it into graphene in precisely defined tracks, one micrometer apart. These are the electrodes. In between we leave untreated graphite oxide, which conducts ions but not electrons and so can serve as an excellent dielectric between the positive and negative graphene electrodes. To complete the cell, we top off the pattern with a droplet of gel electrolyte to provide a bit of battery-like storage—the same technique that’s used in conventional supercapacitors.
By “interdigitating” the electrodes to resemble interlaced fingers, we greatly extend the interface, which increases the surface area to which electric charge can cling. At the same time, we shorten the path over which ions in the electrolyte need to diffuse. That’s important because supercapacitors store charge via the adsorption of ions on the surface of graphene, and therefore the ion diffusion rate controls the rate of charge and discharge of the supercapacitor. Faster ion diffusion means faster charge and discharge capabilities. As a result, the new interdigitated supercapacitors demonstrate greater charge storage capacity than their stacked counterparts.
No photomasks or expensive clean rooms are required for the processing of these microsupercapacitors. This single-step laser-writing method can produce devices at a fraction of the cost of conventional microfabrication techniques. In our lab we can now produce 100 of the devices on a disc in less than 30 minutes, and there is plenty of room for improvement. Of course, a manufacturer could speed things up by simply running a roomful of DVD burners in parallel. It would be even better to optimize a burner for mass production with industrial-scale laser engravers, which are now widely used in industry to mark products so that they can be tracked later on. The laser engraving machines can be constructed on a conveyor-belt system using long rolls of graphite oxide.
The result is a marvelously compact, two-dimensional device that can be integrated directly with silicon circuitry. By contrast, today’s computer motherboards require complex interconnects between the electronics and the backup power supply, typically a coin-size lithium battery that keeps memory alive when system power is off. And because they can be integrated on-chip, these microsupercapacitors could make it easier to extract energy from mechanical, thermal, and solar sources. For instance, they could be fabricated on the backs—literally—of solar cells to store power generated during the day for use after sundown. Typical practice today is to use batteries for this application, but supercapacitors would be better because they can extract charge much more efficiently and with minimal losses. In addition, integrated supercapacitors can simplify the external wiring used in conventional energy harvesting and storage systems.
Ions Go Up & Down, Electrons Go Back & Forth
Electrodes [gray] consist of stacks of chicken-wire-shaped graphene, which conducts electrons. Positive [red] and negative [blue] ions flow between and through the electrodes, completing the circuit of the battery-like element of the supercapacitor.
Our design also sidesteps one of the main challenges in today’s power supplies: leaking electrolyte. Both batteries and conventional supercapacitors use highly corrosive liquid for this function, and as the devices age, this liquid sometimes escapes to eat away at circuits and surrounding components. The result is failure and sometimes even fire. Our microsupercapacitors employ an all-solid-state electrolyte, which we apply directly onto the interdigitated pattern.
For this solid electrolyte, we have plenty of choices. We can use gelled polymer electrolytes, made by swelling a polymer matrix with an electrolyte solution, or we can solidify ionic liquids by adding polymers or silica nanopowder. This nonleaking design, together with a virtually unlimited number of charge and discharge cycles, means that our supermicrocapacitors will likely outlast all other electronic devices on the chip. Such long life will be particularly useful whenever it is inconvenient or dangerous to open things up to replace a power source, as in pacemakers, defibrillators, and other medical implants.
Another interesting feature of our direct laser-writing technique is the ability to link any number of microsupercapacitors together to produce modules with both high voltage and high current output. That beats yoking many cells together with clumsy wiring. More important, we can pack far more high-voltage supercapacitors into a very small volume than any scheme could accomplish today.
Recently, our team has produced a hybrid that combines the best features of capacitors and batteries. We fabricate this hybrid device by growing manganese dioxide—a battery-like material—inside the corrugated graphene structure of our supercapacitors. The hybrid can be recharged in minutes, yet it has an energy density up to 10 times that of commercially available microbatteries. The hybrid device is only one-fifth the thickness of a sheet of paper; its footprint can vary from a few square micrometers up to the centimeter scale. The centimeter-scale devices would have capacitances in the range of 400 to 1,000 millifarads—easily enough to power an LED flashlight for an hour.
Because of their power and compactness, our microsupercapacitors will open up new opportunities. You could knit them right into the fabric of an adhesive bandage so that it could put out a bit of electrical current to stimulate the slow, steady release of a drug. Or you could integrate them onto smart cards to provide an independent, onboard source of energy that could be tapped to erase stored data in case of fraudulent use.
Commercial applications of our devices are now being explored by Nanotech Energy, a Los Angeles–based startup. As mass production expands, unit costs should plummet until microsupercapacitors find their way into camera phones, RFID tags, and solar cells. And as Moore’s Law begins to apply in full force, supercapacitors will begin to shrink right out of sight. As electronics engineers well know, there’s plenty of room at the bottom [PDF].
This article originally appeared in print as “Introducing the Micro-Super-Capacitor.”
About the Authors
Richard B. Kaner is a chemistry professor at the University of California, Los Angeles. Maher F. El-Kady is a graduate student there.