At the beginning of 2014 when a Boeing Dreamliner aircraft caught fire due to the lithium in the rechargeable batteries igniting, we were all reminded that Li-ion batteries have some fundamental safety issues.
One alternative, the magnesium-ion battery, doesn’t present the same safety risks. However, it has been a real struggle to create electrodes for magnesium batteries that don’t fail quickly.
Now researchers at the Department of Energy’s Pacific Northwest National Laboratory (PNNL) are looking at an alloy made from tin and antimony that may hold the secret to making the magnesium-ion battery a more viable alternative. This tin/antimony alloy has been identified as an attractive material for the electrodes of magnesium-ion batteries with a theoretical capacity of 768 milliamperes per hour per gram.
In research described in the journal Advanced Materials, the PNNL researchers peered into this tin/antimony alloy with a transmission electron microscope to see how it behaved and whether it could serve a way to make magnesium-based electrodes easier to produce and longer lasting.
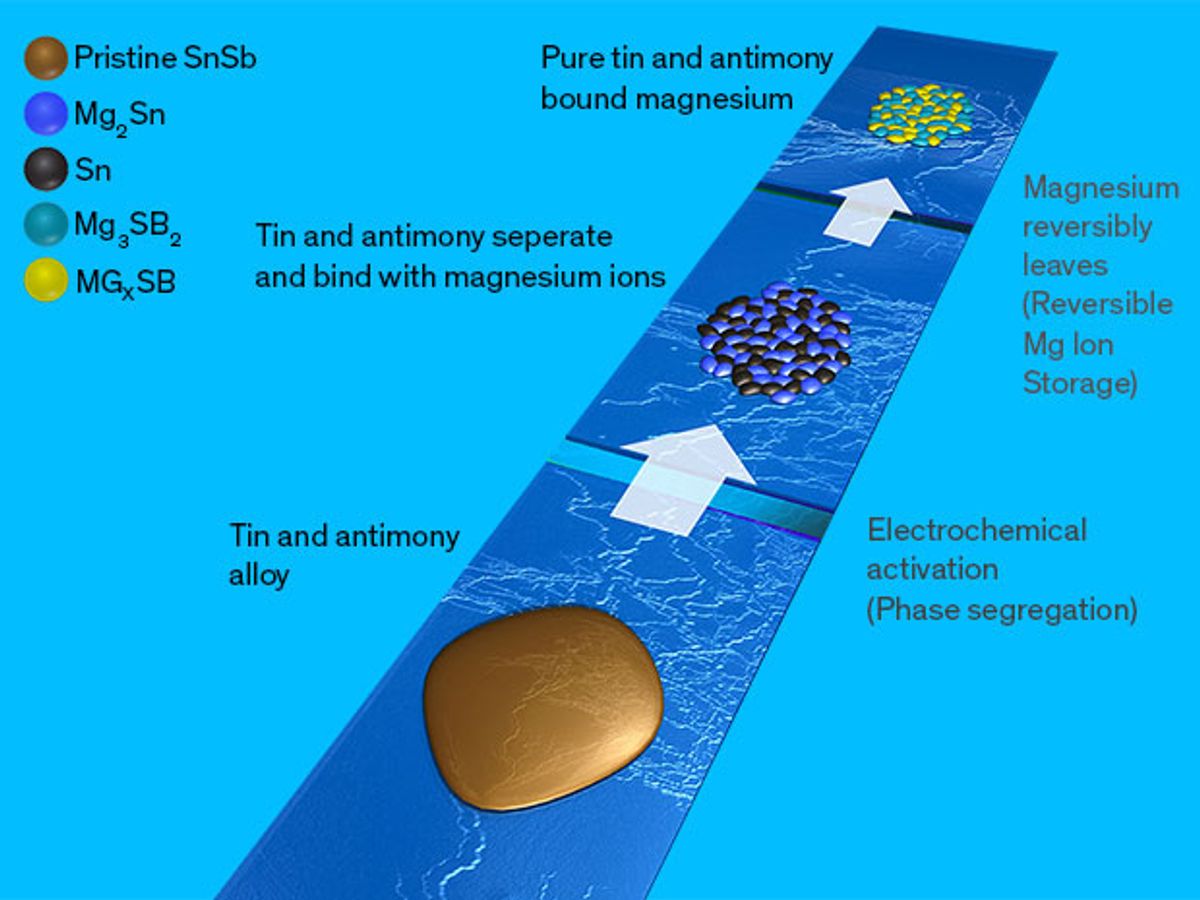
The PNNL researchers discovered that once joined together in an alloy, tin and antimony particles form porous networks of tin-rich and antimony-rich subparticles. The tin-rich regions work well as an electrode material; the antimony-rich areas not so much.
Nonetheless, the antimony regions play a key role in making the alloy effective as an electrode material by keeping the tin from collapsing at the interface between two materials.
In the operation of a magnesium-ion battery that employs this alloy, the magnesium ions would move into the tin region and then later leave. Without the antimony there, the tin would just collapse after the magnesium ions departed. While the antimony can’t really receive or discharge the magnesium ions—which makes it a poor electrode material—it does make the tin a much better one.
"This research offers important information on how to start with an alloy and create a phase-separated material with a completely different structure that is useful for energy technologies," said Yuyan Shao, one of the PNNL researchers, in a press release.
While this research could help usher in the use of magnesium-ion batteries, the researchers also think it provides a better understanding of how alloys work together to enable new properties that the individual materials lack.
Dexter Johnson is a contributing editor at IEEE Spectrum, with a focus on nanotechnology.