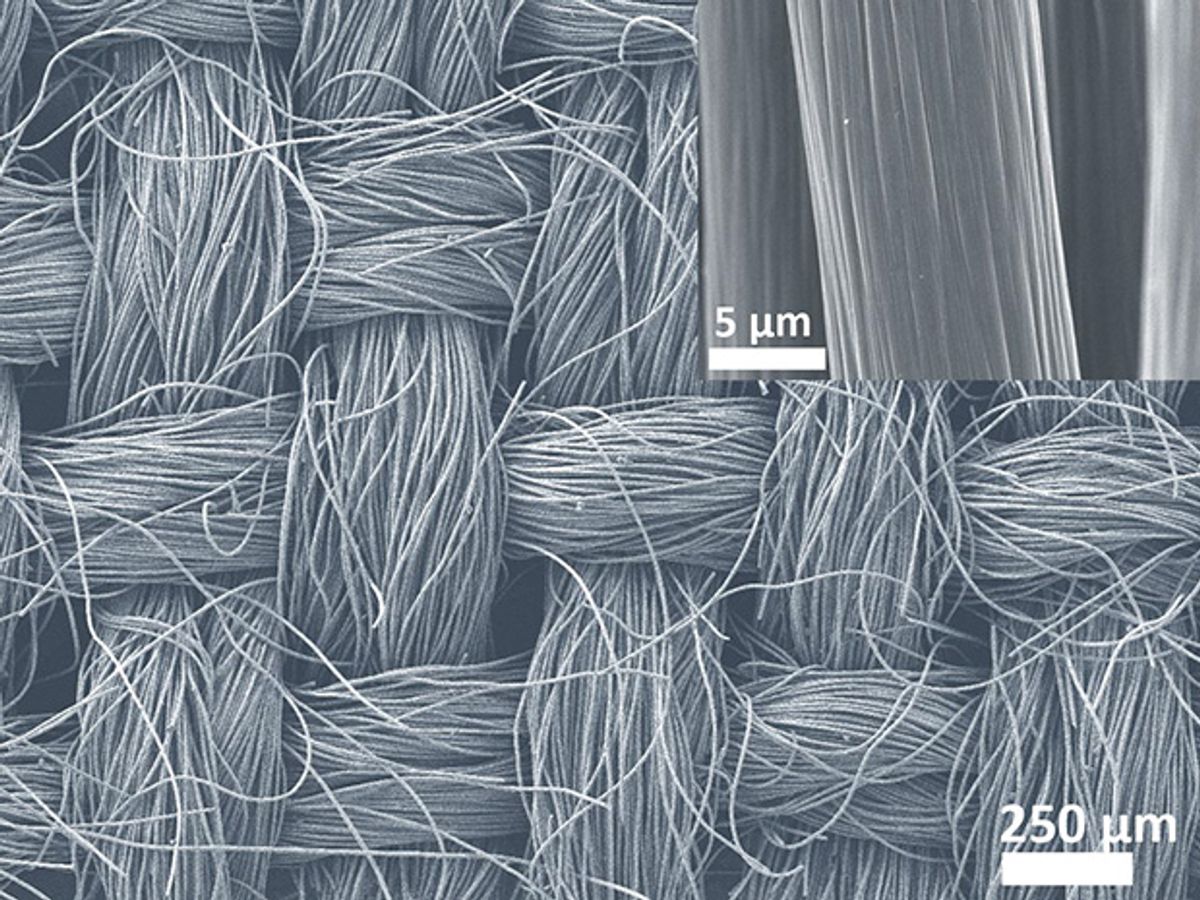
Splitting water can yield clean-burning hydrogen fuel, but catalysts that generate hydrogen are often expensive, and unstable in water. Now a team from Singapore and Taiwan have shown that carbon fiber cloths coated in inexpensive catalysts can generate hydrogen, and perform not only in water but in seawater as well. The researchers detailed their findings online August 21 in the journal Science Advances.
The most effective catalyst for generating hydrogen is platinum. However, this metal is scarce and expensive, limiting its use in large-scale hydrogen generation. Instead, the researchers investigated molybdenum sulfide as a catalyst. Molybdenum and sulfur are respectively about 300 and more than 100,000 times more abundant than platinum.
"One hundred grams of pure molybdenum metal costs $44, while the same amount of platinum costs $3,211.86 today," says study co-author Bin Liu, a materials scientist at Nanyang Technological University in Singapore.
Normally, molybdenum disulfide is bad at generating hydrogen. However, prior studies found that while its flat surfaces are not catalytically active, its edges are. The researchers incorporated nickel into the catalyst, which essentially punched holes into the molybdenum sulfide, increasing the amount of its exposed edges.
The researchers synthesized the catalyst onto carbon fiber cloth, a supporting material that is flexible as well as physically and chemically stable. The carbon fibers are also highly conductive, helping electricity flow as part of the hydrogen-generating chemical reactions.
Hydrogen-generating catalysts often require acidic solutions to release protons. However, this new catalyst can generate hydrogen while in water. It also requires only 200 millivolts to produce the gas, and can even operate in seawater.
The new material generated pure hydrogen roughly as efficiently as other state-of-the-art catalysts, the researchers say. Moreover, the new catalyst was more than six times more stable in water than platinum catalysts were after an hour of activity.
Liu notes that non-precious metal catalysts are more efficient at generating hydrogen. The advantage that molybdenum disulfide has is that it is already widely used in industrial applications such as lubrication and petroleum refining, and that techniques for its mass production already exist. This makes it one of the most promising candidates for sustainable large-scale hydrogen production, he says. The flexibility and mechanical strength of the cloth suggests it could get easily integrated into existing hydrogen gas generators, Liu adds.
The researchers noted that hydrogen generation is only half of what is needed to split water — oxygen generation is necessary also. They are now working on a material to efficiently generate oxygen in water, Liu says. In addition, the researchers will try improving hydrogen generation further by widening the spaces between fibers in the cloths and replacing nickel with other elements, he adds.
Charles Q. Choi is a science reporter who contributes regularly to IEEE Spectrum. He has written for Scientific American, The New York Times, Wired, and Science, among others.