Eight Technologies for Drinkable Seawater
Desalination takes too much energy, but emerging technologies will help
Desalination—removing dissolved minerals from seawater, brackish groundwater, or treated wastewater—appears at first glance to be the ideal answer to freshwater shortages. What could be more attractive than harnessing the planet’s seemingly inexhaustible 1.34 quadrillion (that’s 15 zeros) megaliters of seawater? Oceans are drought-proof. In theory, so is wastewater.
In the Persian Gulf, where seawater normally contains a lip-puckering 45 grams of salt per liter, thermal desalination is king. This method vaporizes water to purify it. Pretty much everywhere else, desalination is done by reverse osmosis, which purifies water by forcing it through membranes. Both methods cut down the salt to a drinkable 0.5 gram per liter.
But there’s a price. As long as the cost of desalination continues to depend on the cost of energy, these technologies won’t help much of the energy-starved developing world that needs them the most. And desalination isn’t just energy intensive; there’s also the problem of the toxic sludge it leaves behind. In this business, brine—the concentrated result of purifying water—might as well be a four-letter word. Throwing the brine back into the ocean can kill fish and smaller denizens of the food chain.
Several contenders might make history of these concerns. New methods in the pipeline reduce desalination’s energy demands in innovative and intriguing ways. Further off are technologies that could turn desalination’s Achilles’ heel into a source of strength: In the future, desalination might just be powered by the very stuff it filters out.
Today
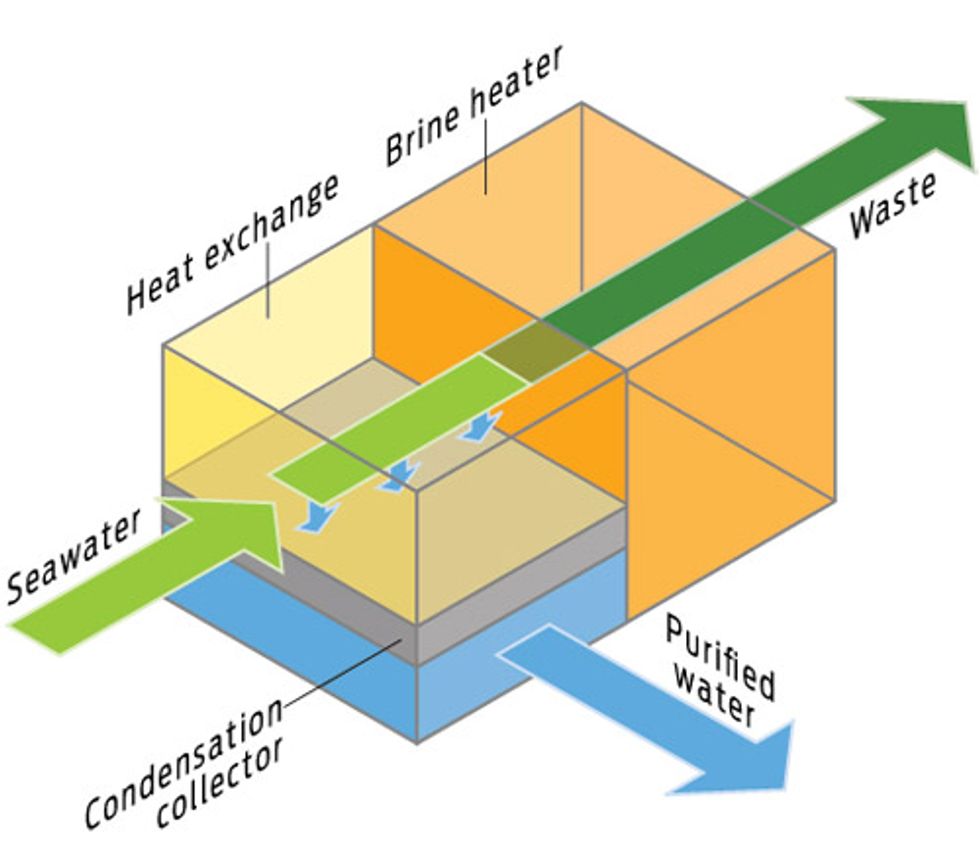
BRUTE FORCE: THERMAL DESALINATION
How it works
Boil seawater, condense the vapor, and drink the result.
Upside
Pure water no matter what the feedstock is.
Downside
Energy intensive, enormous infrastructure, toxic brine waste.
Best for
Ocean water. The energy required is the same regardless of the water’s salt concentration, so the process is well suited for highly saline waters.
Energy trade-off
Evaporation and pumping can consume up to 80 megawatt-hours per megaliter of water produced. Its use is largely confined to the Middle East, where plants tend to use waste heat from adjacent power plants and cheap oil instead of electricity. Thermal desalination is not used in the United States: The typical U.S. energy price of $0.065 per kilowatt-hour translates to $5200/ML of water. At just over half a cent per liter, that’s cheap enough for bathing and drinking, but for irrigation or industrial uses, the price tag is staggering.
What’s next
Saudi Arabia’s Shoaiba, an 880-ML-per-day power and desalination plant on the Red Sea, is the largest thermal desalination plant in the world. But even for seawater, the market-research firm Lux predicts, thermal desalination will not extend past the Middle East, which has the lion’s share of cheap oil to desalinate its very salty water.
Today
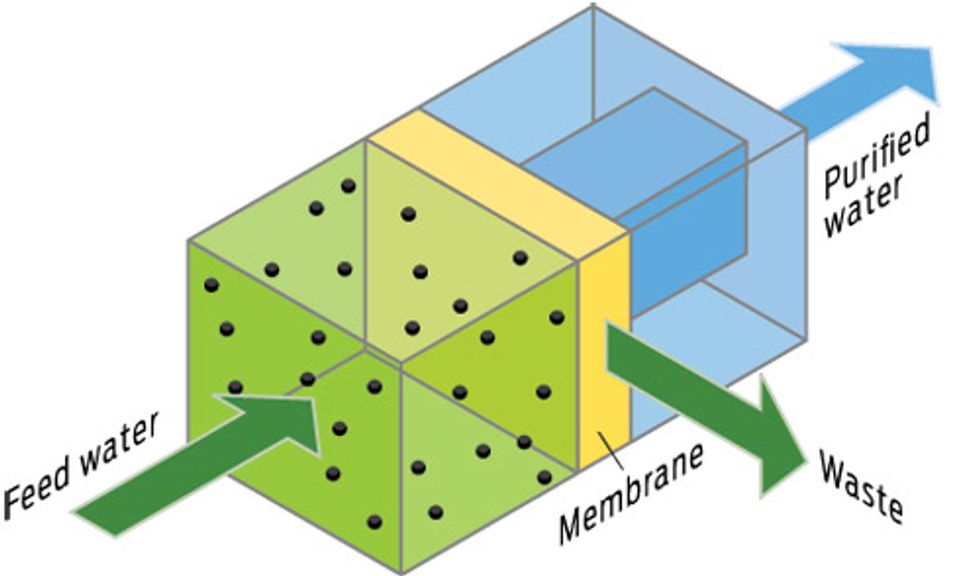
PUSH! REVERSE OSMOSIS
How it works
Separate saltwater and freshwater with a membrane that blocks salt ions, and the freshwater rushes to the salty side by the natural process of osmosis. Reverse osmosis (RO) uses hydraulic pressure to shove water molecules in the opposite direction, with the membrane holding back the salt.
Upside
Comparatively low energy cost.
Downside
Toxic brine; can’t completely filter potentially harmful substances like boron, arsenic, lithium, and some pharmaceutical compounds. When membranes become clogged, they must be scraped and bleached or they stop working; cleaning, however, reduces the expensive membranes’ lifetime. Pretreating the water to remove the gunk slows the rate of fouling but requires a lot of real estate.
Best for
Brackish groundwater, which contains on average only 3 to 5 grams of salt per liter. RO is also increasingly being used to desalinate seawater, however.
Energy trade-off
The pressure needed to push water through the membrane is proportional to the water’s salinity. Higher pressure means higher energy cost. On average, RO demands at least 3.5 MWh/ML produced from brackish water and more for seawater.
What’s next
A Carlsbad, Calif., plant slated to open in 2012 will produce more than 189 ML of drinking water per day from the Pacific Ocean, making it the biggest RO plant in the United States.
In the Pipeline
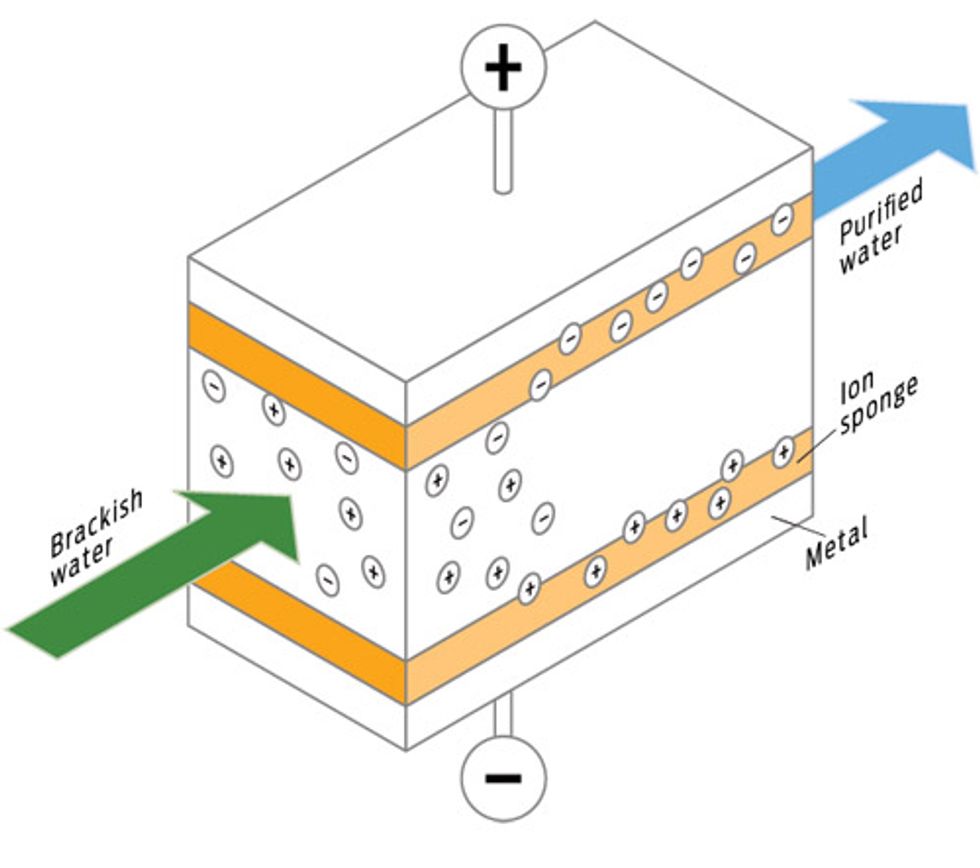
ZAP ’EM: ELECTRICITY INSTEAD OF MEMBRANES
How it works
Capacitive deionization works without membranes. It filters impurities by streaming the water between two charged electrodes. The electrodes attract ions in the water, which stick to them, leaving freshwater. The attached ions eventually clog the electrodes, but cleaning is easy: Simply reverse the electrical polarity to flush the ions back out. Good candidates for electrodes are advanced materials such as carbon aerogel and mesoporous carbon.
Upside
Easy to clean; requires less power. The process could theoretically go on forever without changing electrodes.
Downside
Works only for brackish water; in practice, electrodes can foul. Does not mitigate toxic brine.
Best for
Brackish water.
Energy trade-off
Far less pressure means less energy.
What’s next
This year, a test reactor will be unveiled in New Mexico, part of an international project led by Campbell Applied Physics, in Rancho Murieta, Calif., and several U.S. national laboratories.
In the Pipeline
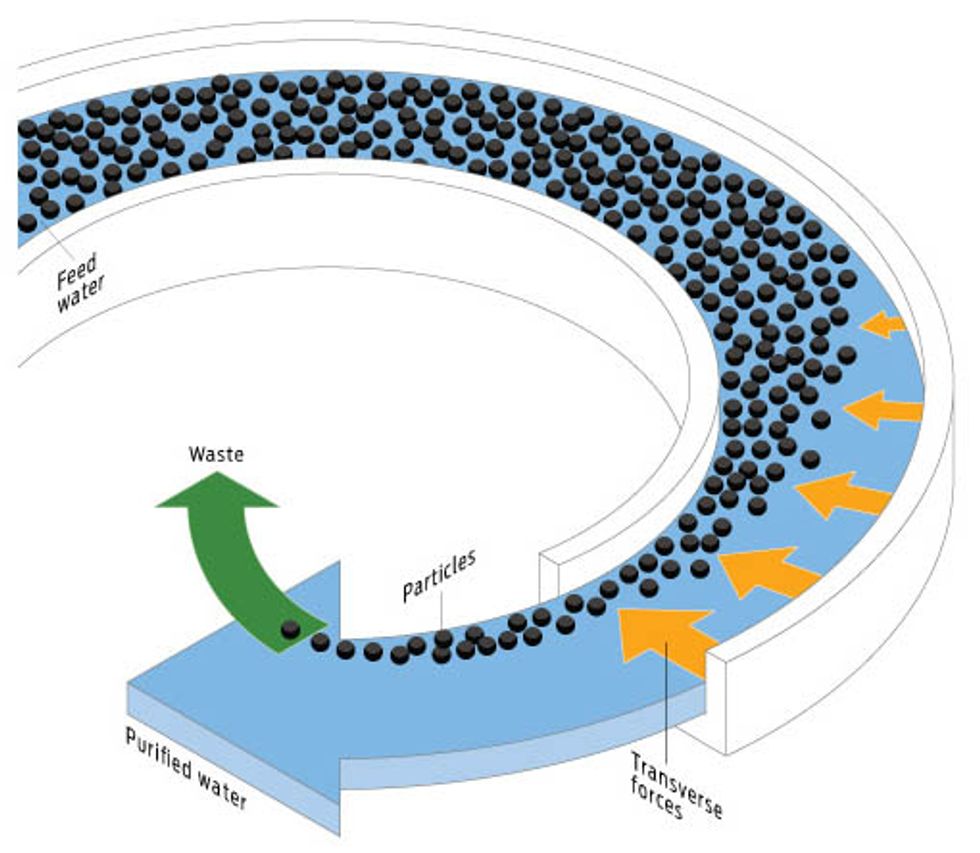
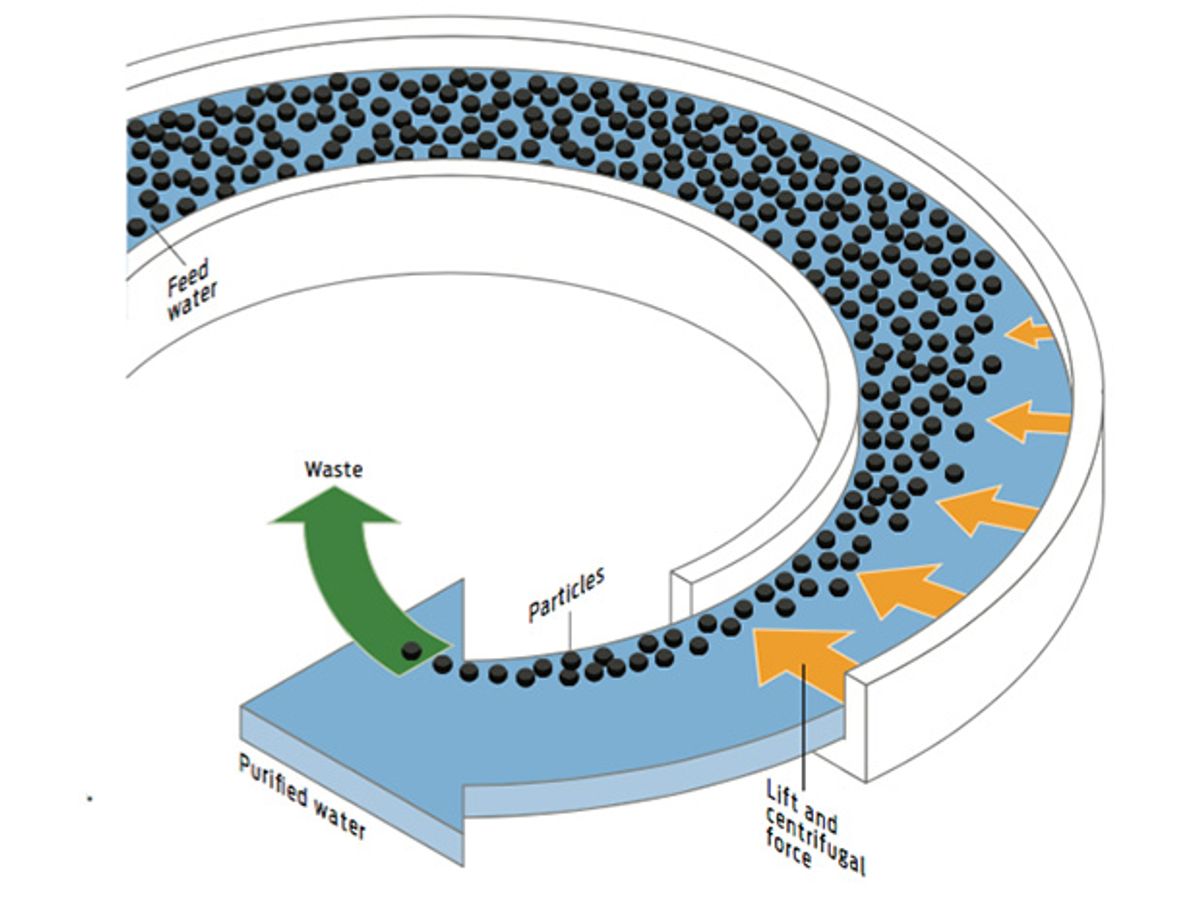
SPIN CYCLE: BETTER PRETREATMENT
How it works
A team of researchers at Palo Alto Research Center (PARC), in California, has developed a reactor that separates particulates from water by sending it through a long, curved channel. Hydrodynamic forces cause the particles to become concentrated near the inner wall of the channel, which allows for easy separation.
Upside
Uses very little energy; compact; reduces the footprint of RO pretreatment.
Downside
The channels themselves might be susceptible to fouling. Does not address desalination’s toxic brine output.
Best for
Seawater, brackish water, and wastewater.
Energy trade-off
Unlike centrifuges, which spin contaminants out of water using g forces in the thousands or tens of thousands, PARC’s laboratory-scale system requires g forces in the single digits. That reduces the energy demand of pretreatment to only 2.5 kWh/ML of water.
What’s next
A test reactor will be installed on a U.S. Navy aircraft carrier later this year.
In the Pipeline
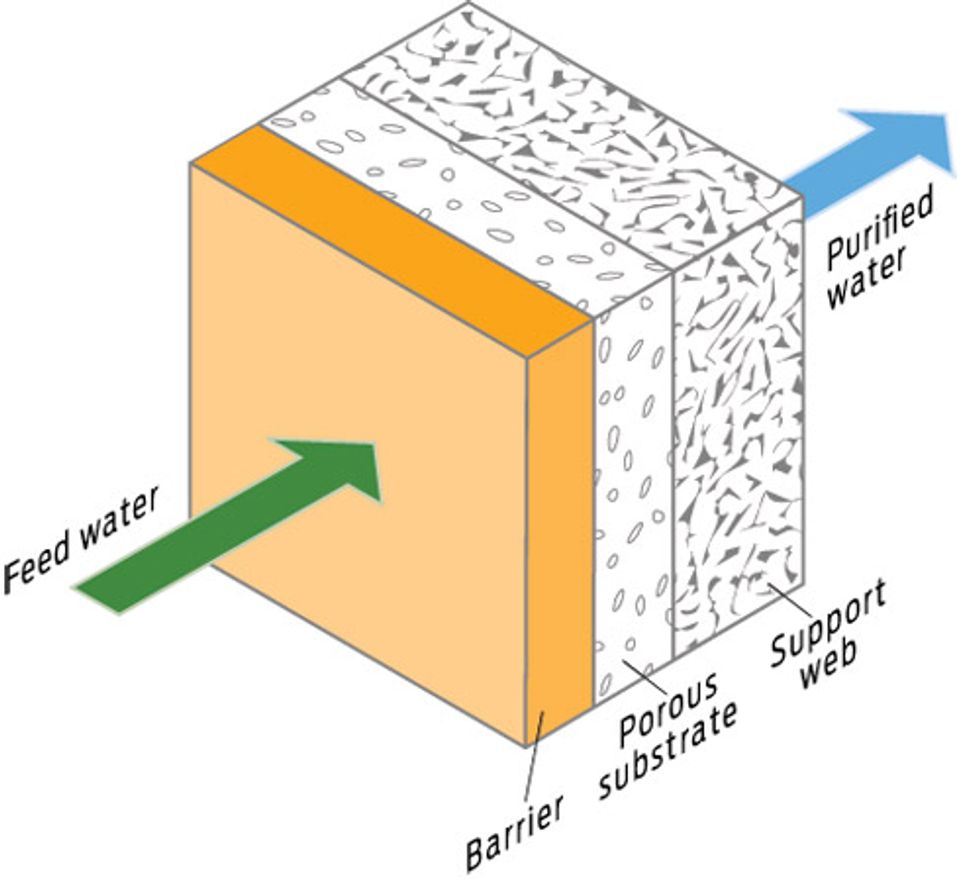
BORDER PATROL: BETTER MEMBRANES
How it works
The next generation of membranes will rely more on clever chemical engineering and less on brute force to push water molecules across them. Less hydraulic pressure means the new membranes would reduce RO’s energy demands. At IBM Research in Almaden, Calif., researchers are working on membranes that will be at once tougher and more sophisticated, permitting only substances with proper molecular identification to cross the barrier. First on the chopping block are arsenic and boron.
Upside
IBM’s membranes show a 90 percent reduction in boron concentration.
Downside
Not yet commercially available. Doesn’t address the problem of toxic brine.
Best for
Wherever RO is used now.
Energy trade-off
Membranes that work at lower pressure should slash RO’s energy demand.
What’s next
In March, IBM Almaden and the King Abdulaziz City for Science and Technology, in Saudi Arabia, announced a joint effort to build a solar-powered water desalination plant using Almaden’s membrane work. The plant will produce 30 ML per day.
Uncharted Waters
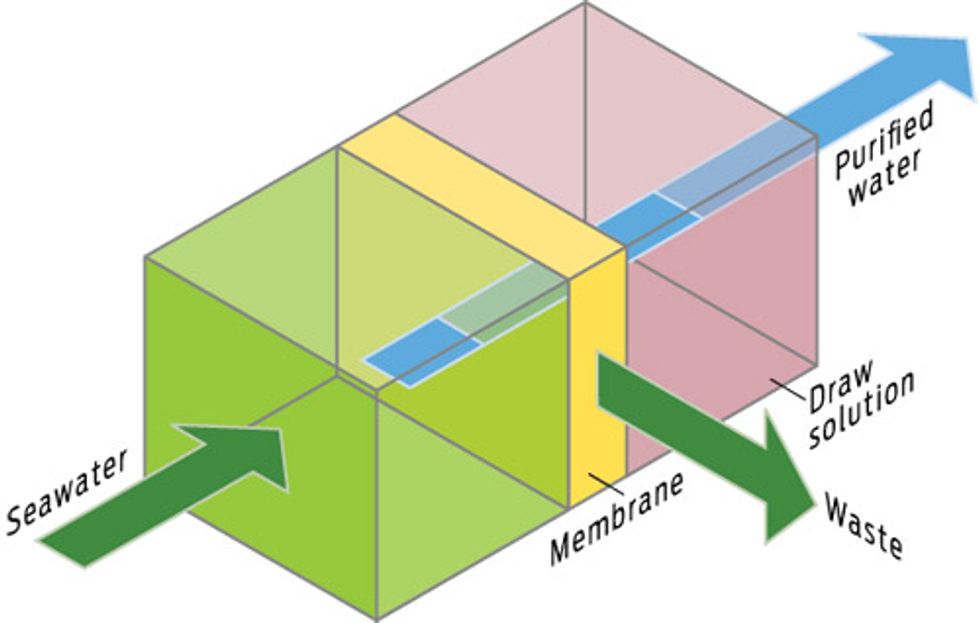
PULL! FORWARD OSMOSIS
How it works
Yale University spin-off Oasys, headquartered in Cambridge, Mass., has developed a method that places seawater next to an even more concentrated salt solution, separated by a membrane. The concentrated solution, a mixture of ammonia and carbon dioxide, sucks the freshwater out of the seawater. Then heat drives off the ammonia and CO2, leaving freshwater.
Upside
Requires very little energy. In theory, the draw solution can be recycled indefinitely.
Downside
Toxic brine waste. The ammonia solution must be completely removed from drinking water.
Best for
Any salt concentration.
Energy trade-off
Oasys says the technology uses one-tenth of the energy required by today’s RO plants.
What’s next
The start-up received US $10 million in investment funding in 2009 and is now working on a commercial platform.
Uncharted Waters
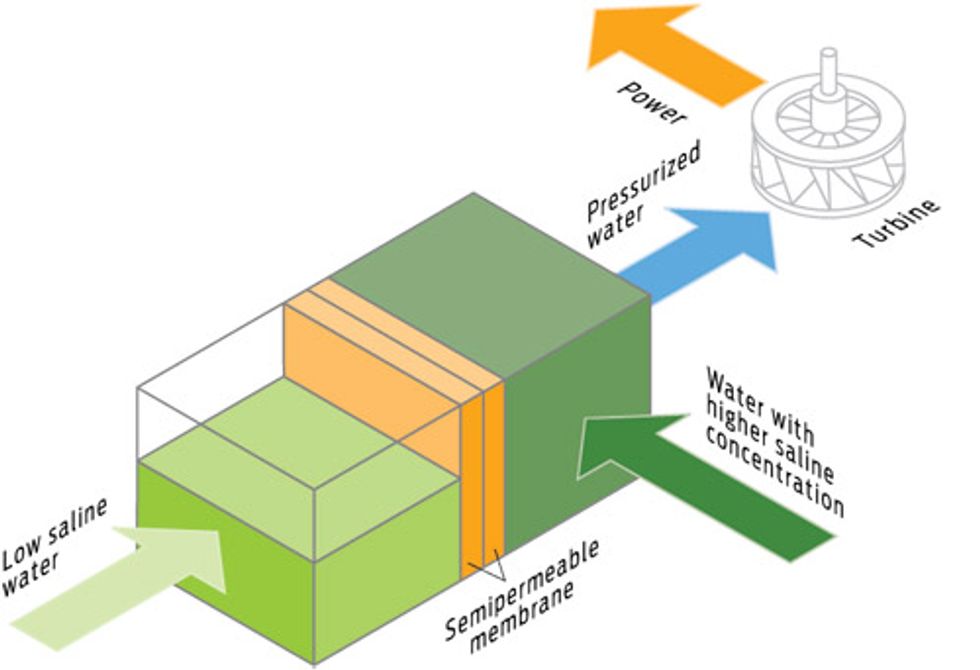
DIFFERENCE ENGINE: OSMOTIC POWER
How it works
Osmosis could be the foundation of a new power-generation method. Separate two sources of water with very different salt concentrations—for example, river water and ocean water, or salty brine and nonsaline wastewater—with a thin semipermeable membrane. Water will rush to the salty side by osmosis, increasing the pressure on the salty side. The resulting pressure difference can drive a turbine and generate electricity. A Norwegian company called Statkraft is doing just that. Its engineers think osmotic power could potentially provide 1700 terawatt-hours a year. This method could even repurpose the brine waste of desalination plants. “Desalination plants produce extremely salty water as the by-product,” says Spike Narayan, IBM Almaden manager of science and technology. “So you have concentrated brine, and you have wastewater coming in with no salt. Bring them together and you can generate energy while you dilute the brine.”
Upside
Generates electricity as it dilutes toxic brine waste.
Downside
The pilot plant produces only enough power to run a coffeemaker.
Best for
Anywhere rivers meet oceans or where desalination and wastewater treatment plants can cross their streams.
Energy trade-off
Coupled with a desalination plant, osmotic power could generate 3 watts of electricity per square meter of membrane, partly recouping some of the energy cost of desalination.
What’s next
Statkraft’s 10-kW-capacity pilot plant began operating in November 2009 in Tofte, near Oslo. The company says it can have a full-scale plant operating by 2015.
Uncharted Waters
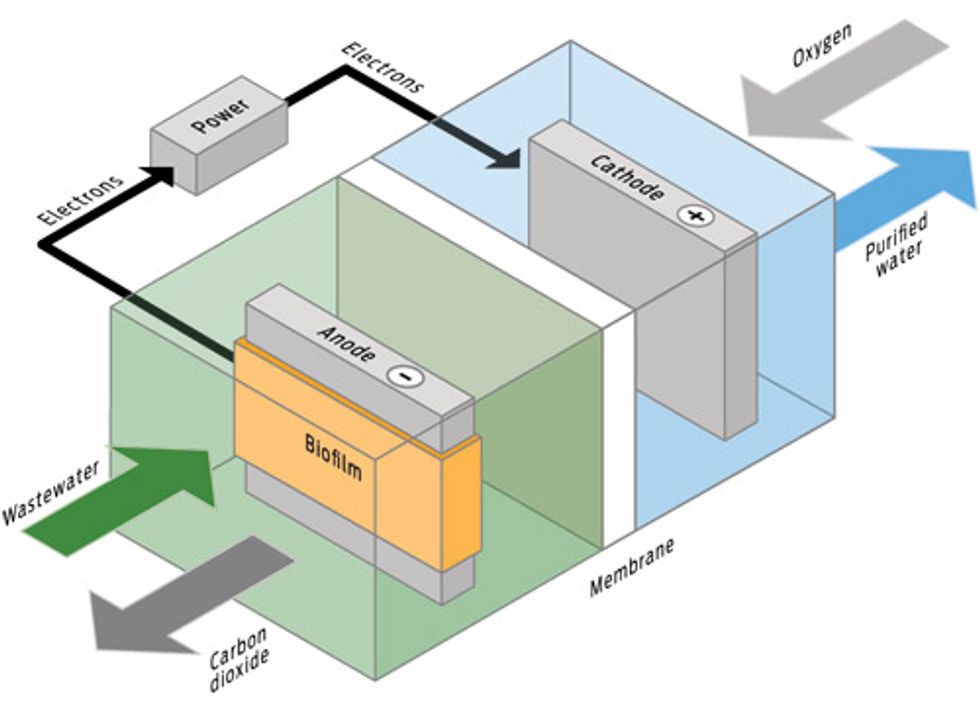
BUG POWER: MICROBIAL FUEL CELLS
How it works
Microbial fuel cells can generate power by cleaning contaminated salty water. The bacteria eat salt and other substances in the contaminated water, and as they clean the water, they generate excess electrons as part of their metabolic processes.
Upside
Self-cleaning; generates power for desalination.
Downside
Still in the lab.
Best for
As yet, this technology is being studied only for wastewater and brackish groundwater.
Energy trade-off
Will generate rather than consume energy—if it works.
What’s next
Research on microbial fuel cells is ramping up around the world, including at the University of Queensland, in Brisbane, Australia, and Tsinghua University, in Beijing. Engineers at Oak Ridge National Laboratory, in Tennessee, are working on combining these fuel cells with a capacitive-deionization reactor.
This article originally appeared in print as “The Saline Solution.”
To Probe Further
Check out the rest of the special report: Water vs Energy.