Neuroscientists Wirelessly Control the Brain of a Scampering Lab Mouse
With wireless optogenetic tools, neuroscientists steer mice around their cages
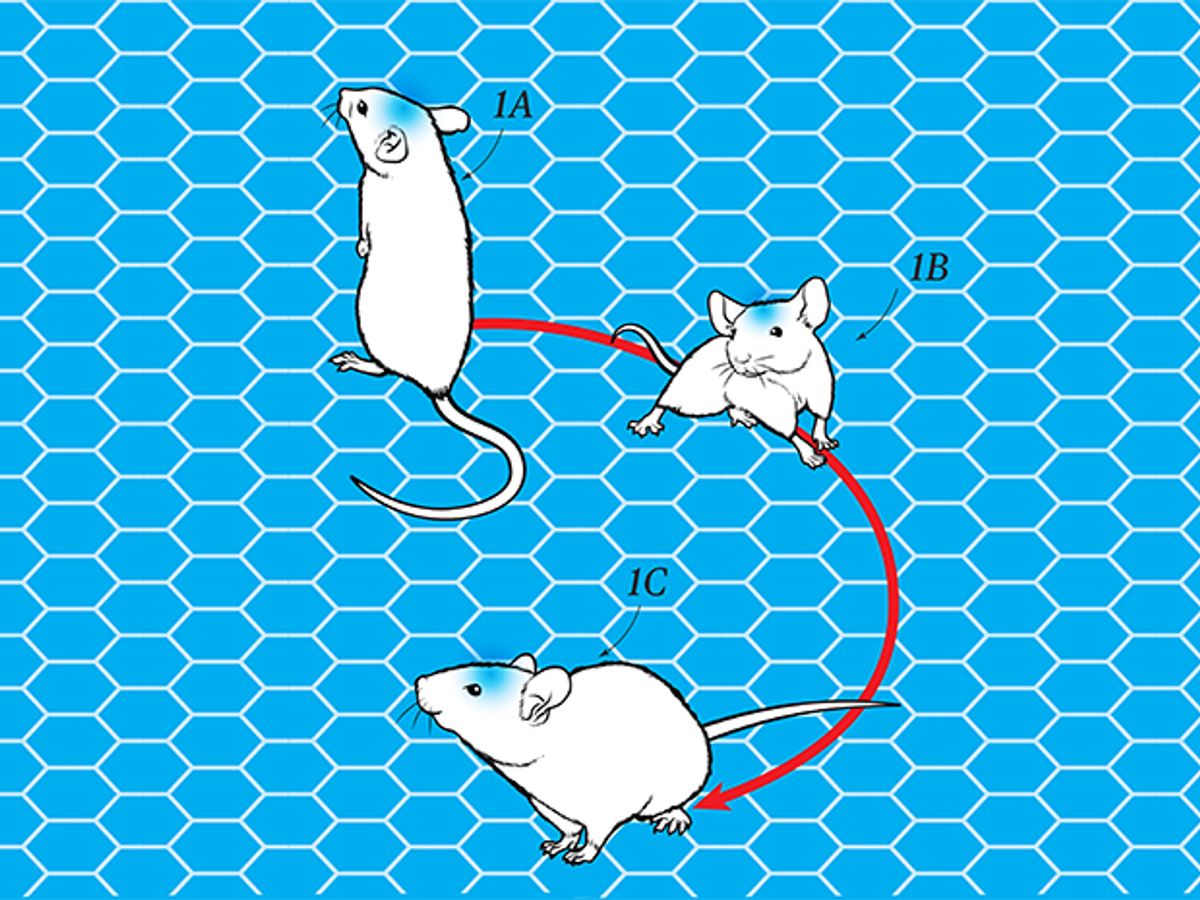
Scientists typically control the behavior of a lab mouse by enticing it with food or repelling it with puffs of air. When I gathered with my colleagues in a Stanford University lab, however, we had a more direct way: We took command of its brain with a shining light.
Implanted in that mouse’s brain was a device about the size of a peppercorn. When we used our wireless power system to switch it on, the device glowed with a blue light that activated genetically engineered brain cells in the premotor cortex, which sends signals to the muscles. We watched in amazement as the mouse stopped its random motions and began to run in neat circles around the cage.

This kind of direct mind control once belonged in science fiction. But with the new technology of optogenetics we can use light to turn on brain cells and activate specific neural “circuits,” allowing us to observe the effect on an animal’s biology or behavior. The goal of such investigations is to find benefits for medicine, both through better understanding the nervous system and, perhaps, through clinical use of the technique. Optogenetics hasn’t yet been tested in human brains, but neurologists could theoretically use light-based stimulation to identify and fix malfunctioning circuits in the human nervous system.
These promising applications have been delayed by the difficulty of getting light into the brain. Some researchers have responded to the challenge by using tiny fiber-optic cables that penetrate the skull and snake through brain tissue to deliver pulses of light. Others have experimented with implanted LED devices powered either by bulky batteries or by clunky head-mounted devices that send power through the skull.
My experiment with the circling mouse proved there’s a better way. My lab develops electronics that can be seamlessly integrated into the body: That peppercorn-size device is about 1 percent the size of any previous optogenetic device. Our mouse sported no cables, batteries, or exotic headgear, so it could move freely, a requirement for common behavioral studies involving mazes or swim tests. What’s more, if other mice were to encounter our experimental one, they probably wouldn’t notice anything unusual about it—opening the way to a host of experiments about social behavior.
Building the tiny device itself was relatively easy; the hard part was figuring out how to efficiently power it while the mouse roamed freely within its enclosure. Our surprising solution used wireless power in the form of radio waves that emanated from a resonant chamber beneath the cage and were captured by the mouse’s own body. With this answer to the optogenetic technology problem, I hope neuroscientists can shine more light on the dark mysteries of the brain.
Have you ever noticed how a houseplant’s leaves gradually swivel to face a sunny window? A similar botanical trick is the foundation of optogenetics.
The trick comes from a unicellular green algae that can swim toward a light source, thanks to a special type of protein on its cellular membrane. The protein responds to light by opening an ion channel in the membrane, thus changing the electrical potential within the algae cell and triggering the movement of two whiplike flagella. Around 2005, several research groups realized they could take the gene that codes for this protein and insert it into the DNA of a neuron.
Optogenetics 101
This cutting-edge technique for neuroscience research begins with a component drawn from a simple unicellular organism
A certain algae species has a light-sensitive protein on its cellular membrane.
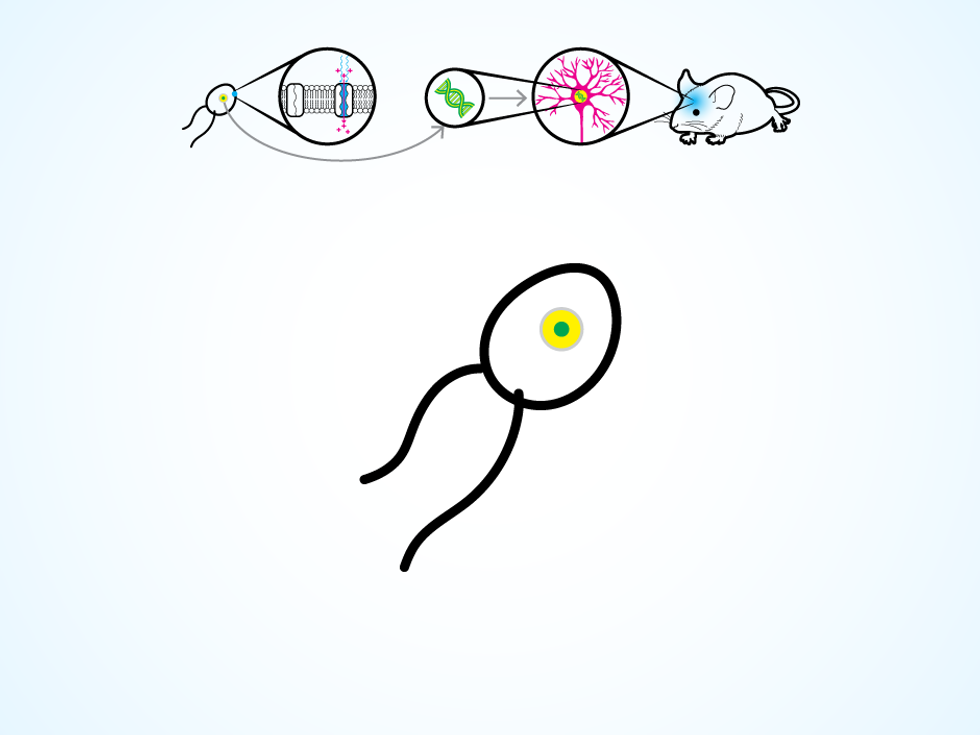
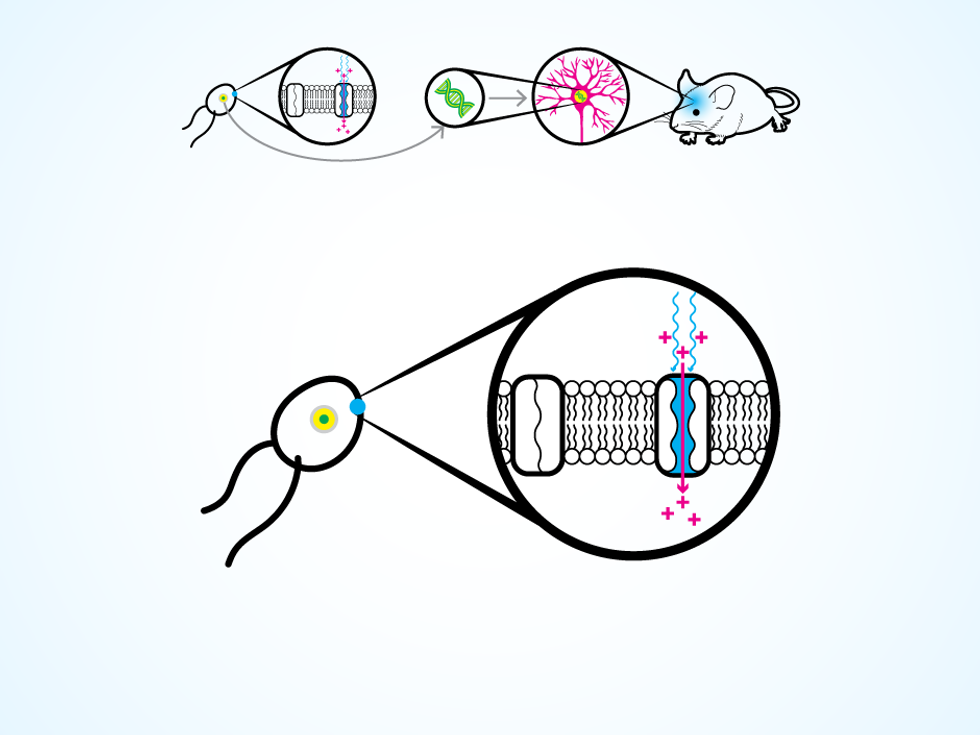
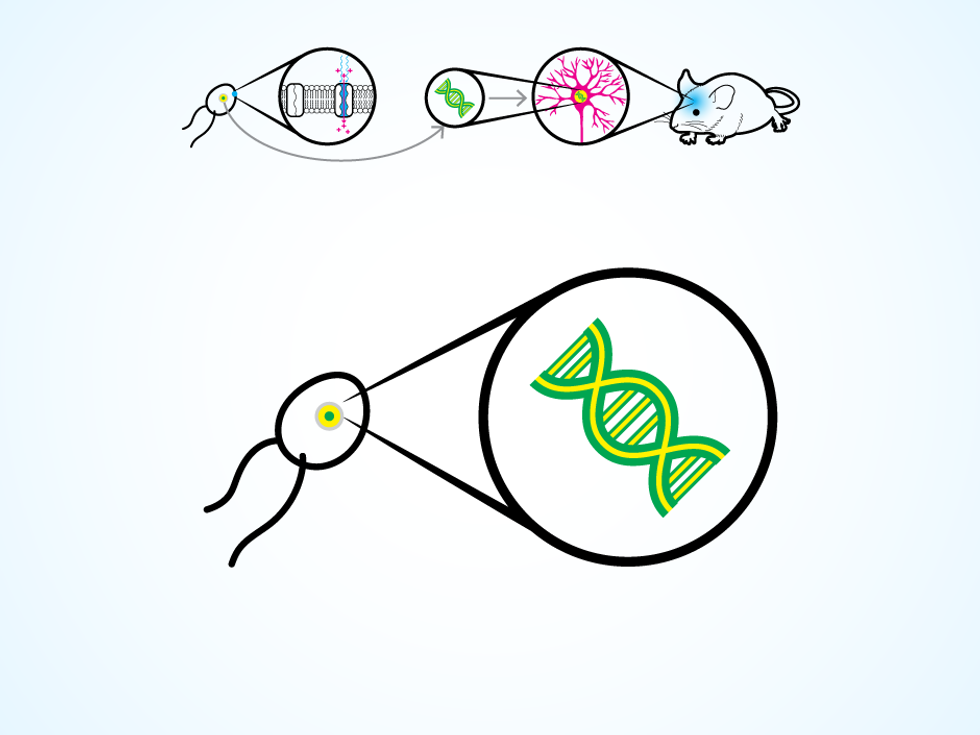
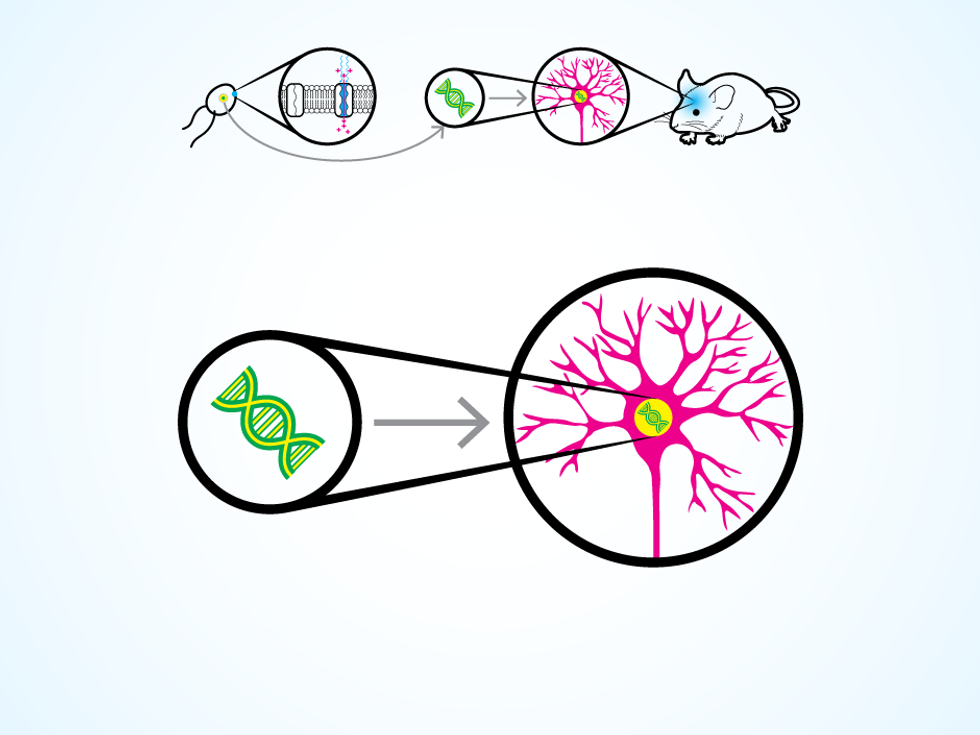
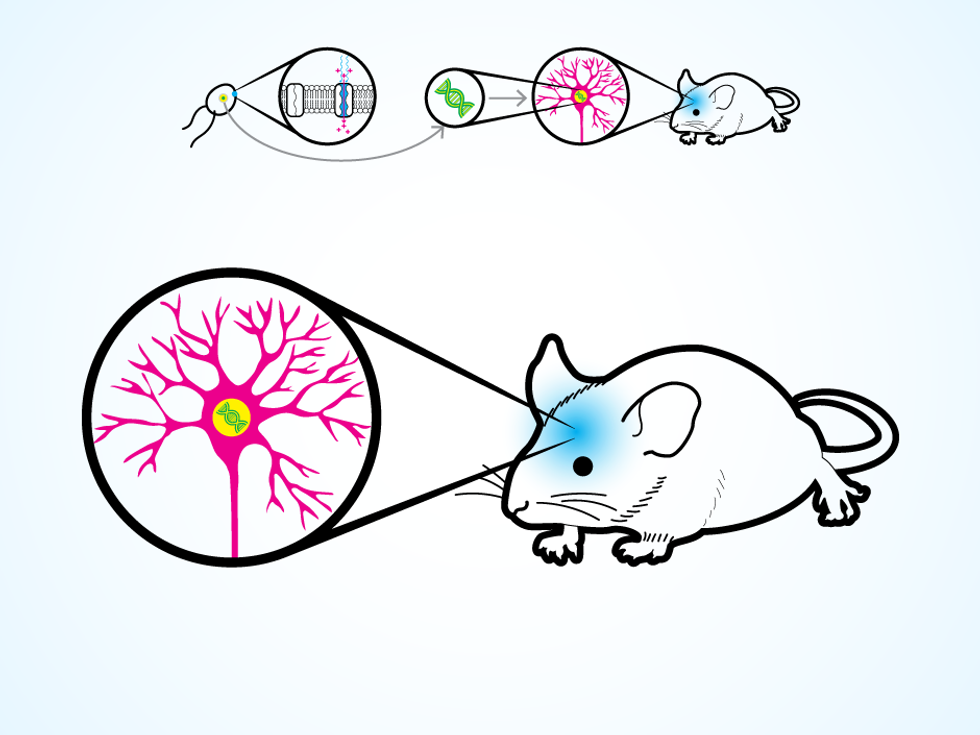
A neuron thus modified produces the protein for its own cellular membrane. Then, when a flash of light hits the neuron, the protein opens up ion channels, letting charged molecules flow in. The process replicates the natural change in electrical potential that makes a neuron “fire” and release a chemical, which causes other connected neurons to fire in turn. The work of the nervous system is done through these chain reactions, as electrical signals flicker through neural circuits to control the operation of organs, voluntary movements, and those mysterious things we call thoughts and emotions.
Neuroscientists study these patterns of electricity, but they’ve been limited by the imprecision of their tools. Much progress in biology depends on observation, which means scientists need tools to both meddle with an organism’s natural bodily systems and watch what happens. Typical neuroscience techniques rely on electricity, using electrodes on the scalp or implanted inside the brain to stimulate and record from groups of neurons. These electrodes are relatively large and crude, though, and can’t target very specific cells, such as the neurons in the hippocampus that encode distinct memories.
This limitation bothers me. From an engineer’s point of view, the study of living creatures can seem messy. When I’m tinkering with an integrated circuit, I can swap out one transistor and check to see if the chip still works. If it doesn’t, I can be sure the new transistor is responsible for the glitch. In biological systems, it’s far harder to isolate a variable of interest.
With optogenetic technology, we can turn neurons on and off as if they were transistors in a circuit. Geneticists have various ways (which we won’t go into here) to deposit the necessary genes into very specific clusters of cells. With our light-up devices, we can then switch on a particular set of neurons. The neurons react to light within milliseconds, making the result of our tinkering fairly obvious.
Neuroscientists have been quick to see the possibilities for studying both healthy brains and those changed by disease. For example, one research group recently delved deep into a monkey’s brain to stimulate only those neurons that produce the neural chemical dopamine, which plays a critical role in movement control, motivation, reward, and addiction. Meanwhile, another group is determining how damage to certain dopamine-producing cells is involved in the movement problems seen in Parkinson’s disease. Although these studies are being conducted in animals, they can inform medical treatments for people.
The first generation of optogenetic technologies used optical fiber to carry a light pulse through the skull, benefitting from the stable skull-brain interface and enabling researchers to consistently light up targeted neurons. In this setup the “tethered” mouse can move fairly freely in an open cage, but the system has its downsides. Investigators must handle the mouse to attach the optical fiber, which stresses the animal and can alter the outcome of behavioral experiments. In addition, a tethered mouse can’t navigate enclosed spaces or burrow into a pile of sleeping cage mates.
Removing the wires posed a formidable challenge. Some researchers tried implanting LED devices with onboard battery packs, but they were too big and heavy for long-term use. Others kept the implants small, but only by attaching a bulky wireless power transmitter to the mouse’s head. These head mounts hinder the animal’s freedom of movement and change its appearance, potentially preventing it from engaging in normal social interactions with other mice.
Our goal, then, was to build an optogenetic system that would allow a mouse to move freely in a social environment. With such a creature, neuroscientists could examine the brain circuitry involved in movement disorders and neuropsychological problems, potentially providing great insights for medicine.
Some people say laziness is the true mother of invention. We invented our system after looking at the existing ways to wirelessly power an optogenetic implant and thinking, “There must be an easier way.”
Most previous devices sent power into the brain via electromagnetic induction, in which a transmitting coil sends electromagnetic waves through the air to a receiving coil. This is an old idea that Nikola Tesla experimented with in the early 1900s, and which has recently been adapted for the wireless charging of electric cars and smartphones. But this energy-transfer method has major disadvantages. To keep the receiving coil tiny enough to fit inside a mouse brain, the transmitting structure needs to be in close proximity to the animal. Ensuring that the mouse receives its power boost as it moves around the cage is also difficult. Either the system must maintain a strong electromagnetic field that covers the whole enclosure, wasting all the energy not received by the implanted device, or it must aim the field at the moving mouse, which requires tracking the animal as it scampers about.
Researchers have taken on the tracking problem by attaching a radio beacon or location sensor to the mouse’s head or feet, but these systems are quite complex. Some even replicate cellular phone networks, with multiple transmitters placed around the cage to act like tiny cell towers, handing off responsibility as the mouse’s location changes. I worked on the design of indoor wireless systems at both Intel and a startup before coming back to academia, and I know how complicated such systems can be. I wanted a simpler solution.
I looked for the answer in the body of the mouse itself. Every object naturally resonates when hit by electromagnetic waves of particular frequencies, which are determined by the object’s geometry and material properties. An easy way to understand the general principle of “resonant coupling” is the classic example involving an opera singer, an acoustic wave, and a wineglass. As the soprano sings her high notes, the sound waves travel through the air to the glass, causing it to vibrate subtly. If a note has the same resonant frequency as the glass, that acoustic wave is trapped in the material and bounces back and forth. In the movies (and sometimes in real life), this wave is strong enough to shatter the glass.
Feeling the Energy: The wireless power system relies on the principle of resonant coupling to transmit RF energy to the implant inside the mouse. The RF energy is generated at a very specific frequency, chosen because its wavelength will cause the radio waves to bounce around, or resonate, within two elements of the system. First the waves resonate in the precisely sized chamber [1] below the gridded floor [2] of the mouse enclosure, keeping the energy neatly stored. The waves also resonate within the body of the mouse [3]. So every point of contact between the mouse and the floor allows the energy to flow into the mouse’s body and travel through tissue to reach the implant’s receiving coil [4].
A living mouse may seem very different from a wineglass, but the principle holds true. Electromagnetic waves can enter the tissue of the mouse’s body, and waves of a certain frequency will resonate within it. So my team used a computer program to model a mouse’s body, putting in information about its general shape and the dielectric properties of its tissue. We then used the simulator to work out the resonant frequencies of a typical lab mouse. Next we built a “resonant chamber” to amplify and store RF energy at one of those resonant frequencies (about 1.5 gigahertz). We placed the chamber under the cage and hooked up a commercially available RF signal generator.
With this setup, the ceiling of the resonant chamber became the floor of the mouse cage. If we’d left it like that, the chamber would have kept the RF energy trapped. But if we removed its top cover, the chamber would have radiated energy in all directions, and we wanted an efficient way to direct the energy only into the mouse. So we replaced the top cover with an open grid in which the holes were much smaller than the 10-centimeter wavelength of our radio wave. This grid trapped the energy inside the chamber below until a crucial moment.
Or rather, until the many crucial moments: Every time the mouse took a step and pressed a paw to the grid, its body became an antenna that was tuned to the radio signal below. Because the mouse’s body resonated at the same frequency as the RF energy contained just below the grid, the energy escaped the chamber and the electric field moved through the mouse’s body. When it reached the brain and the implanted LED device, it was captured by a 2-millimeter coil, which concentrated the directed energy and used it to power the device. So wherever the mouse moved on the grid, its paws drew in the RF energy, while elsewhere the energy stayed tidily contained. No tracking was required, yet our scurrying mouse stayed powered up.
The device we built for the brain contains a power-receiving coil, circuits, and an LED, and weighs only 20 milligrams (a mouse’s head is approximately 2 grams). It measures 10 cubic millimeters in size. In addition to this tiny brain implant, we also built the first optogenetic devices small enough for implantation in the peripheral nervous system of a mouse, allowing us to stimulate nerves in a mouse’s spine and limbs. That capability could let scientists map the propagation of electrical signals throughout the body.
Our system meets the requirements for many neuroscience experiments that study mouse behavior. It can power a device over an area large enough for standard “open field” experiments, which are often used to test antianxiety drugs. Mice don’t like brightly lit open spaces, so their movements and exploratory behaviors can indicate their degree of comfort or anxiety. Our system can also be used in the “place preference” experiments frequently used in drug testing. To test a pain medication, for example, a mouse is allowed to move freely between a safe enclosure and another where it receives electric shocks; if it spends time in the shocking room, the pain medication must be working. Our system could also be used in home cage experiments so the mice wouldn’t have to be handled by investigators at all, which might ensure more natural behavior.
I want neuroscientists across the world to use our technology, so I published our final designs online, along with a how-to video. The wireless power transmitter is easy to build using readily available tools and cheap components. This system is also far easier to use than prior wireless optogenetic systems, as it requires no tracking mechanisms or custom equipment beyond the resonant cavity.
I hope this ease of use leads researchers to adapt our device to meet their needs. Our present technology works with small animals that we’ve modeled to identify the correct resonant frequencies, but a similar approach may be effective for rats and larger animals. I can also imagine scientists customizing the setup for different environments or adapting it for studies involving multiple interacting mice, each with an LED implant glowing inside its brain.
Everyone always wants to know when optogenetics will be ready for medical use. Since such use would involve genetically modifying cells in the human body, we’ll first need a great deal of safety research to determine whether these modifications have unintended consequences.
However, even if optogenetics stays in the realm of basic scientific research, it can still deliver clinical benefits in the near term. Working with mice that are commonly used to study Alzheimer’s disease, my lab is now deploying our technology to probe the mechanisms of memory loss in early stages of that devastating ailment. We’re also looking for treatments. Our hybrid approach uses both optogenetic and electrical stimulation of neurons in the hippocampus, the brain region involved in memory.
Here’s what we do: With our implanted LED device, we directly activate clusters of neurons until we identify those associated with the mouse’s recall of a very specific memory, such as the fearful experience of receiving a painful shock. It’s easy to tell when the mouse recalls this memory, because it freezes in a defensive crouch. Once we’ve identified the crucial neurons via this optogenetic scrutiny, we can then turn our attention to electrodes that are also implanted in the brain. Guided by the precise information we’ve gathered, we use the electrodes to stimulate the hippocampus more broadly, watching to see which jolts produce the desired outcome.
If we can learn where individual memories are stored and find ways to access them using our suite of tools, our work can directly inform clinical studies. Doctors already use implanted electrodes to treat several neuropsychiatric diseases, so lessons learned in the lab can be transferred to the clinic. We’re aiming high: We hope that by revealing the stimulation patterns that cause Alzheimer’s mice to recall a shock, we’ll help scientists discover similar patterns that cause people with Alzheimer’s to remember more useful things, like the events of their lives or the faces of the people they love. As optogenetic technology gets better and makes delicate brain research easier, I believe it can be a guiding light for neuroscience.
This article appears in the December 2016 print issue as “A New Kind of Wireless Mouse.”
About the Author
Ada Poon is an associate professor in Stanford University’s department of electrical engineering.



