Brain-computer interfaces have managed some amazing feats: allowing paralyzed people to type words and move a robot using only their minds, to name two examples. Brown University neuroengineering professor Arto Nurmikko has had a hand in some of those developments, but even he says the technology is at only a rudimentary stage—the equivalent of the computer understanding the brain’s intention to bend a single finger.
“We’re trying to go from the bending-of-the-finger paradigm to tying shoe laces and even to the concert pianist level. That requires lots more spatial and temporal resolution from an electronic brain interface,” Nurmikko says. His team is hoping that kind of resolution will come along with the transition from a single, hard wired neural implant to a thousand or more speck-size neural implants that wirelessly communicate with computers outside the brain. At the IEEE Custom Integrated Circuits Conference, engineers from Brown University, Qualcomm, and the University of California San Diego presented the final part of a communications scheme for these implants. It allows bidirectional communication between the implants and an external device with an uplink rate of 10 megabits per second and a downlink rate of 1 Mb/s.
“We believe that we are the first group to realize wireless power transfer and megabits per second communications” in a neural implant, says Wing Ching (Vincent) Leung, technical director at the Qualcomm Institute Circuits Lab at UC San Diego.
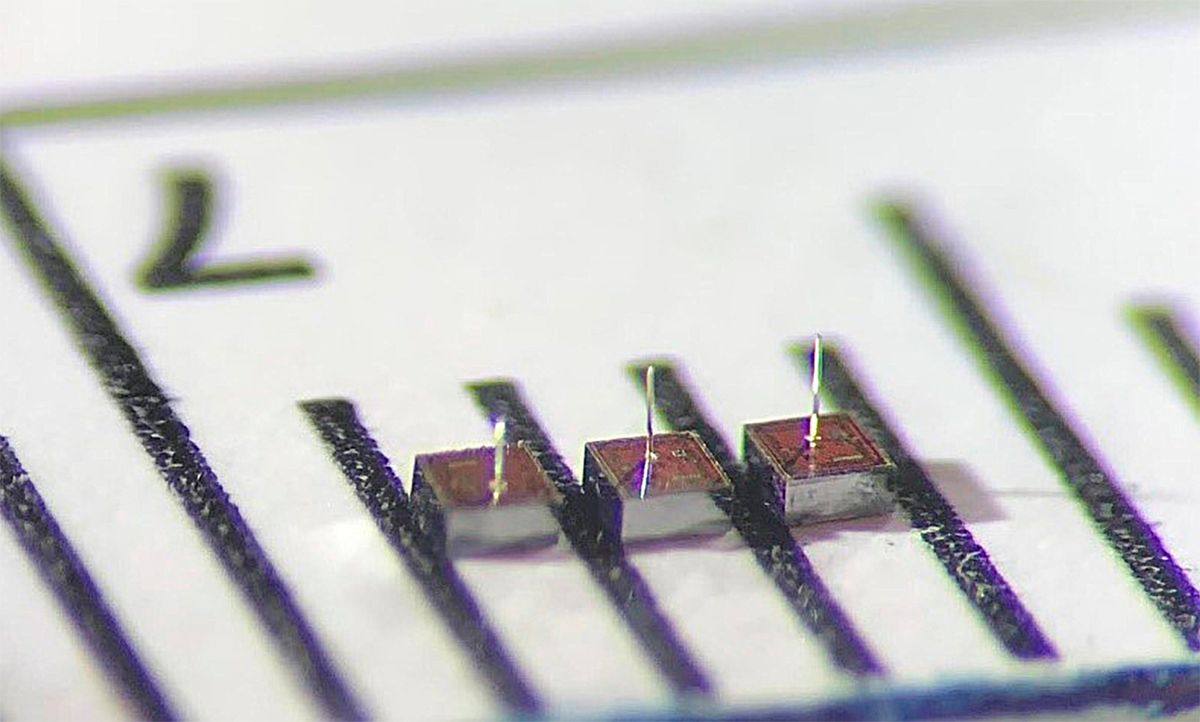
Nurmikko calls the 0.25-square-millimeter implants “neurograins.” They each consist of a chip capable of harvesting RF energy; that chip powers an electrode that senses spikes of voltage from individual neurons, as well as the wireless communications. An antenna set outside of the skull provides the RF power, transmits to the implants, and receives data from them.

Permitting a thousand implants to talk to the same external antenna created some difficult downlink problems for the neurograins team to solve. For one, the neurograins had no way to coordinate their on-chip clocks with each other. For another, they all receive slightly different amounts of power, but they don’t carry a reliable voltage reference for comparing to the highs and lows of incoming bits.
“We had to form a synchronized network with no common reference and no clock between the nodes, and do it with low power and small area,” says Leung.
The answer came in the form of a purpose-built low-power voltage comparator and a communications scheme called amplitude-shift keying with pulse-width modulation. In that scheme, bits are represented as a change in amplitude in a pattern that depended on how long that change lasted. Each bit has both a high and low portion. A “1” has a high pulse that lasts twice as long as the low pulse portion that follows it, and a “0” is high for half as long as the low-amplitude portion that follows. This ensures that the bits are still identifiable even though none of the neurograins’ 30-megahertz clocks are synched together and there isn’t a reliable voltage reference with which to compare their signals.
Leung and Nurmikko’s team had already worked out other aspects of the communications system. Using the new downlink scheme, the part of the system that resides outside of the head powers the neurograins and then addresses each one in series, commanding them to upload data at 10 Mb/s. Under this arrangement, a thousand neurograin implants can all have their say in the space of 100 milliseconds.
The team says it has one remaining task: integrating the neural recording and stimulation circuitry. But even with that addition, Leung expects neurograins to be reduced to 0ne-tenth of their current size, which would make implantation less invasive.
Samuel K. Moore is the senior editor at IEEE Spectrum in charge of semiconductors coverage. An IEEE member, he has a bachelor's degree in biomedical engineering from Brown University and a master's degree in journalism from New York University.