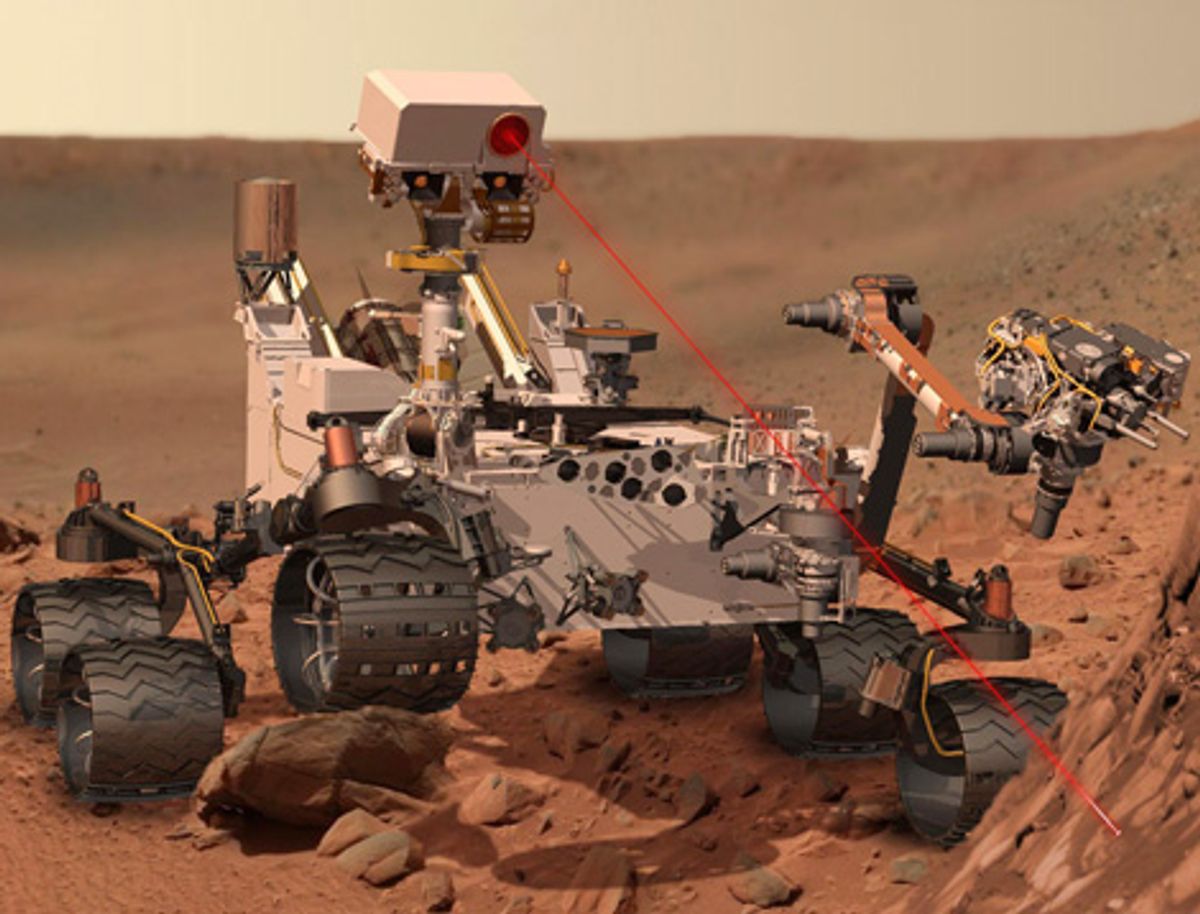
At around 5:25 a.m GMT on Monday, 6 August, the NASA Mars Science Laboratory spacecraft will reach the edge of the Martian atmosphere and attempt to deliver the biggest and heaviest robotic explorer yet sent to the Martian surface. If the Curiosity rover survives the landing, it will be set to scrutinize the Martian surface with the most sophisticated instrument package ever landed on another planet. The expectation is great that the US $2.5 billion mobile laboratory will be able to tell us once and for all whether Mars could once have harbored—or might still be able to support—some form of life. But whatever Curiosity finds, it won’t be the final word on Martian life.
If Curiosity’s mission is successful, it will be at best an incremental step—albeit an important one—in our exploration of the Red Planet. Speculation about the possibility of life on Mars has long outstripped our ability to investigate it, and our technology is only now beginning to catch up. In the years to come, we may look back on Curiosity as the spacecraft that began to answer questions that researchers have been asking, in one form or another, for centuries.
For a long time, we struggled to pin down whether Mars ever had the most basic ingredient needed for life: liquid water. In 1907, after astronomer Percival Lowell claimed to have found evidence of canals built by intelligent Martians, the British naturalist Alfred Russel Wallace wrote: “The first essential of organic life—water—is nonexistent. Mars, therefore, is not only uninhabited by intelligent beings such as Mr. Lowell postulates, but is absolutely UNINHABITABLE.” Later telescope observations supported Wallace’s conjecture, and by the 1960s, when the first spacecraft finally flew past the planet, the pictures they snapped showed scientists a vast desertlike surface, pockmarked with craters—a cold, dry, and desolate world.
Nowadays, we know the truth is more complex. Thanks to data collected by both orbiting and landed spacecraft, planetary scientists now have reason to believe that water once flowed across the surface of Mars and perhaps even formed lakes and small oceans. Liquid water might still exist in isolated spots beneath the surface, in thin melted layers that sit atop subsurface water ice.
That said, it’s still far from clear whether life ever took hold on Mars, or whether some forms of microbial life might be present. Water may be the ideal medium for life as we know it, but it’s just a prerequisite. Evidence of water alone does not mean that life is inevitable. Living things also require carbon-based polymers, such as nucleic acids and proteins, which can carry out fundamental biological functions like replication and catalysis. If we want to establish whether life exists or once existed elsewhere in the solar system, those are the compounds that we need to detect.
Over the years, planetary scientists have had to significantly revise their expectations for what they might find. In 1976, when the first successful Mars landers—Viking 1 and Viking 2—reached the planet’s surface, many had high hopes that the spacecraft’s instruments would be able to settle whether life—even simple microbial life—existed on the Red Planet. Each of the Viking landers carried a suite of instruments, including a combination gas chromatograph/mass spectrometer that was designed to detect organic compounds liberated from heated samples. But none of the Viking instruments found convincing evidence for the existence of life, or even its most basic ingredients.
About 30 years later, NASA’s Phoenix Mars Lander made a discovery that may explain why the Viking spacecraft came up dry. In 2008, Phoenix found abundant amounts of the potent oxidizing agent perchlorate in the Martian soil. Perchlorate can destroy organic compounds released during sample heating, and it may also be able to destroy organic material in the upper layers of Martian soil, well before it is scooped into a spacecraft sample chamber. The surmise is that, if organic compounds exist on Mars, perchlorate may have prevented them from being picked up by Viking’s instruments.
Curiosity could finally settle that question. The rover’s Sample Analysis at Mars (SAM) instrument includes a mass spectrometer and a gas chromatograph, just as the Viking spacecraft did. But each of these instruments now boasts much better capabilities. Working in concert, they will be able to detect trace amounts—at the level of a few parts per billion—of organic compounds released from the stepwise heating of samples retrieved from surface soils and cores of rocks. Although this level of sensitivity isn’t too different from Viking’s, Curiosity will be able to heat the samples up to greater than 1000 °C, twice the peak temperature of the Viking instrument set. That will help liberate materials that might otherwise stay trapped and inert in a sample. What’s more, SAM’s analysis protocol calls for adding substances that can reduce the temperatures at which organic compounds are released from samples. Perchlorate salts are thought to be released only at higher temperatures, and with this procedure, samples will be heated to only 350 °C, which should reduce their tendency to decompose.
All told, if organic compounds exist on Mars, Curiosity could find them. But although such a discovery would be a historic first for planetary exploration, chances are it won’t tell us anything about potential Martian life. Organic compounds such as amino acids—the building blocks of proteins—are present in and produced by all life forms on Earth. But these molecules can also be produced by natural, abiotic processes and are abundant in certain types of meteorites.
There are tests that can differentiate between these two possibilities. You can, for example, measure an amino acid’s molecular structure for its handedness, or chirality, an asymmetry that makes a molecule structurally distinct from its mirror image. Amino acids that form abiotically in nature tend to have a fifty-fifty chance of being either left- or right-handed. But all the life forms we know are based only on left-handed amino acids (although it is possible life elsewhere could be based on either left- or right-handed amino acids). If we find an overabundance of one type of amino acid, we might conclude that it’s linked to life of some sort. Unfortunately, finding an excess of left-handed amino acids may also simply mean that Curiosity brought these amino acids as contaminants from Earth. Finding an excess of right-handed amino acids would be significant because this has never been found before anywhere. One plausible explanation for this excess could be unique Martian life.
Another key test is to measure the isotopic concentration of elements in the organic compounds. Biological processes tend to skew the isotopic composition of materials relative to natural concentrations; the carbon in our bodies is more likely to be made of light isotopes than is the carbon in the atmosphere. If we saw a similar bias on Mars, that would suggest that life is—or was—present. But Curiosity’s SAM won’t be capable of performing either of these tests, so at best we will be left with a tantalizing hint that something exists on Mars that might be produced by life.
We already have one such hint, but it doesn’t come from the surface. For years, planetary scientists have been intrigued by some signs that Mars boasts traces of methane in its atmosphere. Earth’s atmosphere also contains a small amount of methane, which is created mostly by microorganisms. Curiosity’s tunable laser spectrometer will be able to confirm whether methane is in fact present in the Martian atmosphere. But it won’t be able to take the next step—to determine whether the methane comes from Martian biota or from nonbiological sources, such as geochemical processes in the Martian crust.
If Curiosity manages to find organic compounds in the Martian soil and methane in the atmosphere, it will certainly spark some intense discussion about the possibility of extant Martian life. But for all we know, Curiosity’s findings could be entirely negative. If Curiosity finds no evidence of organics or methane, it may simply imply that the surface conditions are just too harsh to permit the survival of organic compounds. Future missions may need to dig well into the subsurface, as much as a meter or more. But for now, we must simply wait and see and hope that Curiosity survives the landing.
About the Author
Jeffrey L. Bada is a professor of marine chemistry at the Scripps Institution of Oceanography at the University of California, San Diego. He led the development of the Urey instrument, which is designed to detect and characterize the properties of amino acids, including their handedness, as well as other organic compounds. The instrument concept may one day be included in a future robotic Mars mission.