Last March, as the Silicon Valley tech community abandoned open offices for working at home, tech entrepreneur Steve Kirsch turned his attention, startup skills, and available cash to finding a cure—or at least a solid treatment—for COVID-19.
Kirsch, the entrepreneur behind the optical mouse, Infoseek, Frame Technology, and, most recently, digital currency startup M10, approached the pandemic the same way he had approached starting companies. Was there a big problem? Yes. Did he have a personal stake in solving it? Absolutely. Did he have a unique, or at least, otherwise neglected approach? That remained to be seen.
“I’m only going to go into something because I see a way to do something better, in a way that’s not being done,” Kirsch says. “That’s why I did the optical mouse. We had mechanical mice, but they didn’t work very well. That was personally frustrating to me, and I had a new approach that I thought would work substantially better.”
Kirsch isn’t new to the medical research world. He’s been funding research projects through his the Kirsch Foundation for more than two decades now. When I interviewed him in 2000, he told me quite seriously that he aimed to improve air quality in California, improve education, reform the U.S. political system, cure major diseases, and save the world. He has funded efforts in many of those areas over the years. But medical research has been his main philanthropic focus since 2007, when he found he had a rare blood cancer: Waldenstrom’s macroglobulinemia. At the time, the average life expectancy for someone diagnosed with the disease was five years. Kirsch believes his support of researchers working on Waldenstrom's has kept his cancer from progressing.
Which brings us back to March, and Covid. After talking to scientists about what was—and wasn’t—being done in the fight against Covid, Kirsch spotted an opportunity in an area he knew something about: funding researchers working on repurposing drugs. Since then, he’s been spending what he says is 18 hours a day talking to researchers, raising money to add to his own funds invested in the effort, and, most recently, pushing the FDA to expedite processing of an emergency-use authorization for fluvoxamine, a widely used antidepressant.

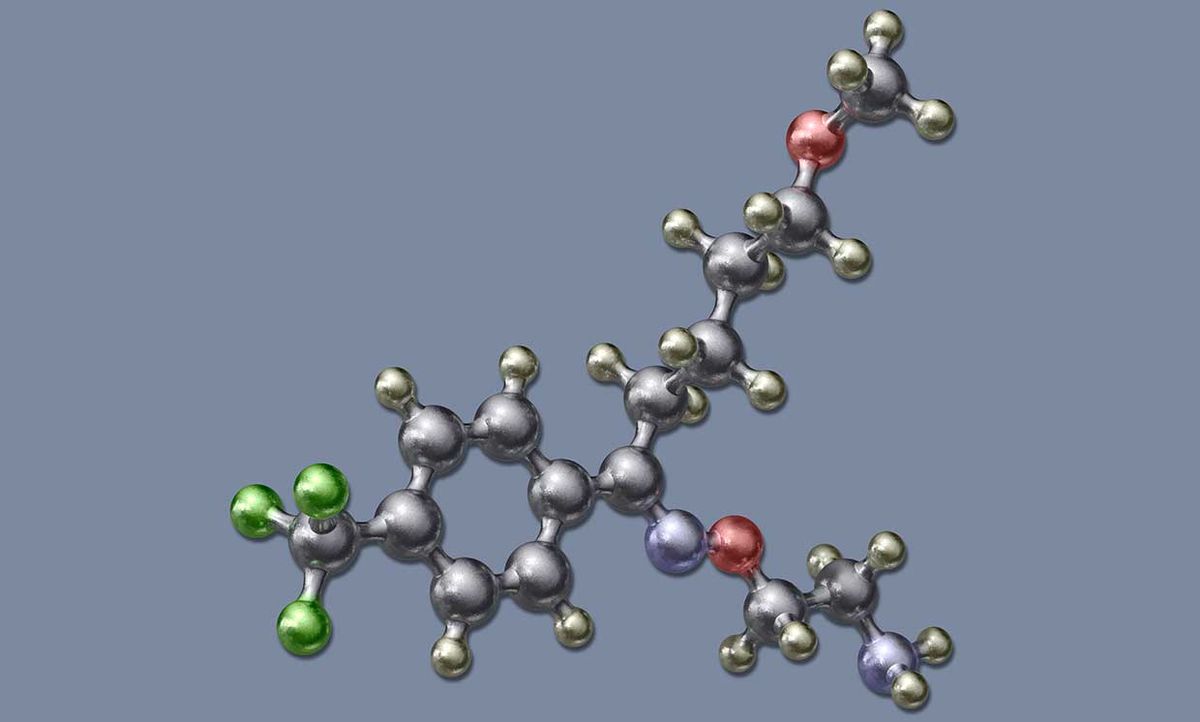
Kirsch believes that fluvoxamine will be a game changer: an inexpensive, easy-to-take pill with few side effects that has proven—in a double-blind, peer-reviewed study published in the Journal of the American Medical Association—to prevent severe illness and death from the coronavirus. (The JAMA paper hypothesizes that fluvoxamine may indirectly regulate small proteins called cytokines, whose uncontrolled proliferation in the body has been linked to severe COVID-19 disease as well as increased mortality.)
Kirsch is engaging in the hunt for Covid treatments with the passion he brought to all of his startups: a relentless energy he’s exhibited since childhood. And as Internet pioneer Vint Cerf—who first encountered a 12-year-old Kirsch on the UCLA campus when Kirsch snuck into a computer room and peppered Cerf with questions—told me when asked why he took the child under his wing, “You can’t buy enthusiasm.”
Indeed, Kirsch’s energy practically crackled from my Zoom screen earlier this month, as he met my questions about his coronavirus treatment hunt with a stream of rapid-fire answers while simultaneously sending backup data via email. I asked Kirsch about his efforts over the past year, why he’s so excited about fluvoxamine, and other Covid research projects that may bear fruit. Here’s what he had to say.
IEEE Spectrum: How did you get started with all of this?
Steve Kirsch: It was the third week in March when we realized that [the coronavirus] is a big problem. I started calling my scientist friends, some who I had funded before, and was referred to infectious disease experts. These experts all said that repurposed drugs would be the fastest and the cheapest way to end the pandemic. I also found that the scientists that were pursuing repurposed drugs weren't getting any funding.
I thought that, for $20 million, we could probably end this pandemic. We could fund all the most promising researchers working on the most promising drugs. And, I thought, it seems like I should be able to raise $20 million quickly from my millionaire friends. I'll put in a million myself, all I have to find is 19 other like-minded people.
Spectrum: That’s the COVID-19 Early Treatment Fund (CETF)?
Kirsch: Yes. So I wrote a check. But it took us about a year to raise just $5 million. The only other million-dollar donors were the Skoll Foundation and the Flu Lab.
Spectrum: So that's the money. How did you find the scientists?
Kirsch: They found us. I tried to advertise, but nobody would print the op-eds I’d written, until, they said, I proved that repurposing drugs works for COVID. We couldn’t get any publicity. It was only because somebody who had heard about us mentioned us in letter to the editor of the New York Times that the guys doing fluvoxamine found out about us. They applied for a grant, we had our scientific advisory board review the paperwork, and we gave them the $67,000 they asked for in about a week. That allowed them to complete their study.
Spectrum: And it was published in JAMA.
Kirsch: Yes, on November 12th. But I had the results on August 24th. They showed a zero-percent hospitalization rate versus an 8.6 percent hospitalization rate in the group that got the placebo.
And we had other papers confirming that the drug really works. There was an earlier paper showing that fluvoxamine was successful in treating sepsis. And there was an observational study in France that SSRIs [like fluvoxamine] are protective [against COVID]. Meanwhile, we couldn't find a shred of evidence that showed that fluvoxamine would not be effective against this disease. At this point, the chance that fluvoxamine doesn't have a major protective effect against COVID is less than 1 chance in 1014.
Spectrum: To date, CETF has reviewed 60 proposals and funded 14. Are any others looking as good as fluvoxamine?
Kirsch: I think that we’ll be able announce results from the camostat mesylate [a protease inhibitor used to treat pancreatitis] trial pretty soon. I expect that those will be quite impressive as well.
Spectrum: But now you’re spending most of your time evangelizing fluvoxamine?
Kirsch: We applied for an Emergency Use Authorization from the FDA in late January. Lately, we’ve been just trying to find out how that’s going, like, ‘What do you guys think? Can we have a conversation? We've got new data.’ But they won’t talk to us, they say it’s in process, that we’ll hear from them soon, likely in five weeks from submitting our request. But five, six weeks have come and gone, and we’ve heard nothing back on our application. Meanwhile, people are dying. If these guys were [working] in Silicon Valley, they’d be fired.
Spectrum: And you got banned from Medium for writing about it.
Kirsch: It’s a Catch-22, you can’t talk about it until it works but it can’t work until you talk about it. I wrote on my Medium blog that fluvoxamine was successful in treating Covid, and that doxazosin [another drug, used to treat high blood pressure among other things] has a 75 percent chance of preventing hospitalization. I was reporting actual results of peer-reviewed studies. In response, Medium removed six years of blogs that I’d written about technology and banned me for life. In my appeal, I said there was no evidence that disputed what I said, and Medium never produced any evidence in response.
Spectrum: You did get covered on 60 Minutes. [On March 7, the news magazine reported on the successful use of fluvoxamine off label to treat a COVID-19 outbreak at the Golden Gate Fields thoroughbred racetrack, with lots of puns about long shots and dark horses.]
Kirsch: Yes, but you could easily watch that 60 Minutes story and believe that we need more data before people should start using fluvoxamine. But a panel of key opinion leaders from the NIH, CDC, and academia met in January and recommended that fluvoxamine be added to the NIH guidelines. They also recommended that doctors should talk to patients about using fluvoxamine for COVID in a process known as "shared decision making." And If you look at the website c19early.com, which rates the drugs with the best evidence, you'll see that the highest-rated FDA-approved drug is fluvoxamine. We shouldn't be ignoring it. Instead of doing nothing, we should be using the drug with the best evidence so far. If you were drowning and someone threw you a life preserver that had only been used 20 times, you don't throw it back complaining there isn't enough evidence that it works.
Spectrum: What now?
Kirsch: Now it’s about getting more trials done, since that is the only way to prove it works to the medical community. We’ve started a phase 3 trial in the U.S., and there is another trial in Brazil with a fluvoxamine arm. The next priority is testing the combination of fluvoxamine with an antiviral . The other priority is to get the word out that this stuff works and is far better than doing nothing.
Today, it's no longer the virus that's keeping us locked down now. It's our inability to assess the less than perfect evidence on the table today and make a decision: Do we save more lives by waiting for more data? Or trying a known safe drug with the best efficacy evidence? There is no question that the latter will save more lives.
Tekla S. Perry is a senior editor at IEEE Spectrum. Based in Palo Alto, Calif., she's been covering the people, companies, and technology that make Silicon Valley a special place for more than 40 years. An IEEE member, she holds a bachelor's degree in journalism from Michigan State University.