The Water Cost of Carbon Capture
Coal power’s carbon savior could double its water woes
Despite all the talk of moving to greener energy sources, coal will be with us for the foreseeable future. It’s just too cheap and plentiful. But if we’re really serious about cutting carbon dioxide emissions, coal plants everywhere will need to substantially reduce the billions of metric tons of CO2 they annually emit into the atmosphere. The big hope is that in the next few years the plants will begin capturing and storing a large portion of that CO2 deep underground, in the oceans, or in mineral form.
But the technology needed to capture carbon has a huge downside: It could nearly double the amount of water a plant uses for every kilowatt of electricity it delivers—easily erasing any gains from techniques aimed at conserving water.
“This technology was not developed in a water-constrained environment,” says Jared Ciferno, technology manager for the existing plants program of the National Energy Technology Laboratory (NETL). “The bottom line is that [carbon] capture takes energy, and that translates to additional water use.”
Just how much water is pretty shocking. By 2030, the addition of carbon-capture technology would boost water consumption in the U.S. electricity sector by 80 percent, or about 7500 megaliters per day, according to research at NETL, which is operated by the U.S. Department of Energy. For plants in water-stressed areas, that’s a deal breaker. “It is not likely that there is enough water supply available to any of our plants to allow for double the water use,” says John Coggins, manager of resource planning at Salt River Project, a water and energy utility in Arizona.
The 80 percent figure assumes that the electricity generation lost to powering the carbon-capture system is made up for by adding more water-cooled coal-fired power. In other words, for a 550-megawatt plant to both capture its carbon and still deliver 550 MW of electricity, it would need to add more than 125 MW of additional generating capability to cover the energy used in capture. If you don’t make up for the lost generation, or make it up in some way that requires no water and emits no carbon—with a wind farm, say—the additional water consumption is more like 40 to 50 percent, according to NETL’s Ciferno.
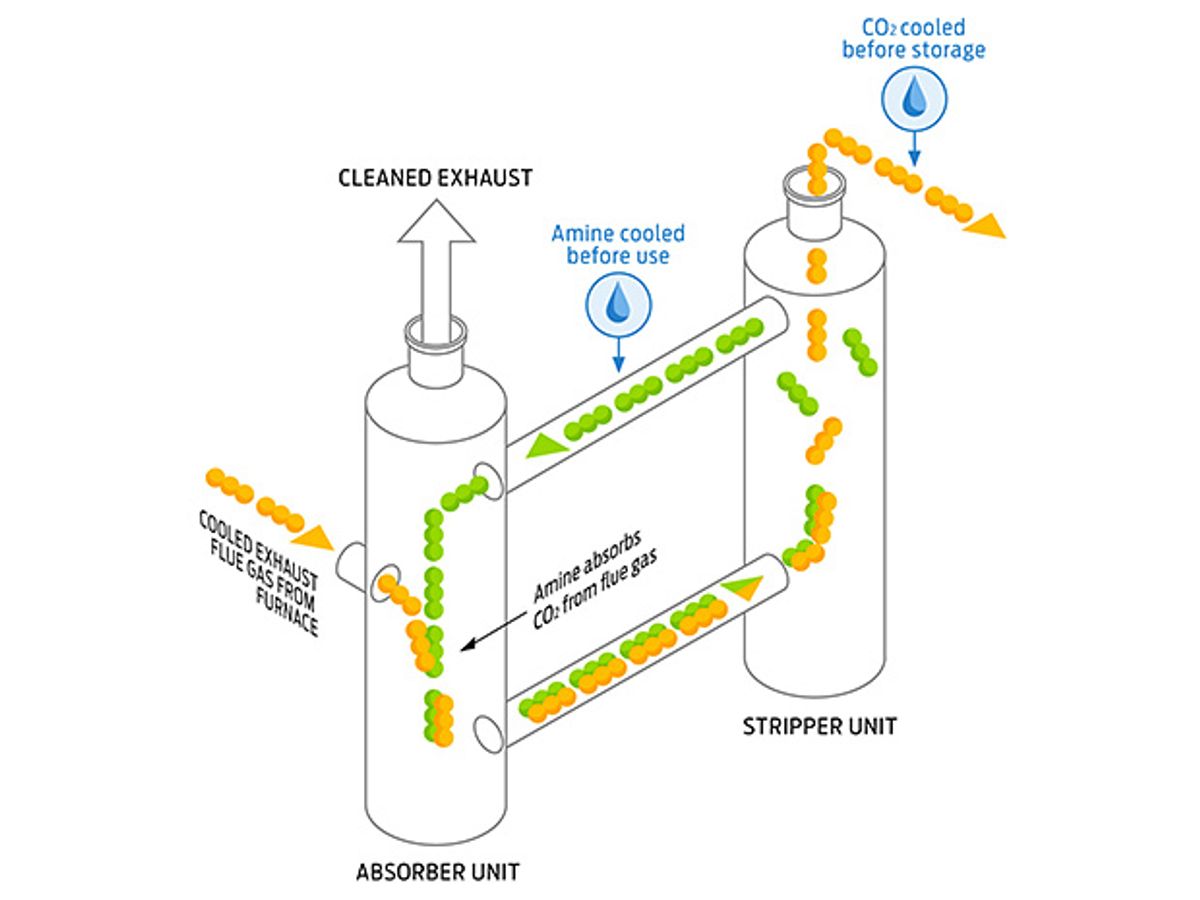
That’s still a lot of water. For coal power plants, the state-of-the-art carbon-capture technology is known as amine-based wet scrubbing [see “Catching Carbon,” above]. It’s basically the technology that puts the fizz in your Fanta. First, the plant’s flue gas is scrubbed of sulfurous nasties; what’s left is a mixture of nitrogen, water vapor, and CO2. An amine solution then reacts with the CO2, yielding a gas stream of mostly nitrogen, which goes out the smokestack, and a CO2-rich amine solution. The solution is heated to strip the CO2 from the amines. The CO2 is then cooled and compressed for storage, and the amines cycle back to pick up more CO2.
Why does this process demand so much water? It’s all about the cooling. The power plant’s cooling tower carries heat away by evaporating water. Cooling the amines for CO2 absorption—which generates heat in itself—leads to an additional load on the cooling tower, causing more water to be lost. And compressing the CO2 to the supercritical conditions needed for storage requires cooling, too. (A less-water-intensive process would be used for integrated gasification combined cycle and oxyfuel plants, but the former technology is used in only a slim minority of generators, and the latter is not yet in operation [see “Restoring Coal’s Sheen,” IEEE Spectrum, January 2008].)
To really reduce CO2 emissions, says Ciferno, less thirsty forms of carbon capture will have to be developed. His lab is now focused on reducing the amount of energy involved, betting that this will take care of carbon capture’s water woes, too. With a budget of about US $50 million per year and 40 projects, NETL has perhaps the biggest R&D program in this area. The goal is commercial-scale technology by 2020 that can capture 90 percent of a coal plant’s CO2 while increasing the cost of generating electricity at that plant by less than 35 percent.
Industrial firms already have several pilot projects capturing small streams of CO2 at plants in Europe and the United States. However, none have yet been scaled up to the size that would make a noticeable difference in a plant’s water consumption. France’s Alstom Power, for one, uses chilled ammonia instead of amines, which the technology company says should be more energy and water efficient. Alstom tested the process last year with a 20-MW pilot plant at American Electric Power’s New Haven, W.Va., generating station. AEP now plans to use it to capture carbon from 235 MW of the New Haven plant’s 1300-MW capacity, starting in 2015.
Germany’s Siemens Energy has also developed an alternative technology, which relies on amino-acid salts instead of amines. Amino-acid salts pick up more carbon than amines do, so you need to pump and cool less material, says Tony DoVale, president of Siemens Environmental Systems and Services. So far the process has been demonstrated to capture carbon while leaching only 9 percent of a plant’s power, compared to amine technology’s typical 20 percent. That “would ultimately imply half the cooling load,” says DoVale.
Of course, unless plant operators are compelled to capture carbon, these energy and water costs won’t be borne at all. “Why would you put on a piece of equipment that puts 10 percent of a plant’s output away if you didn’t have to?” says DoVale.
This article originally appeared in print as “The Carbon Capture Conundrum.”
To Probe Further
Check out the rest of the special report: Water vs Energy.


