Natalia Trayanova has spent years creating computer models that simulate individual patients’ hearts. Now, the Johns Hopkins University engineer is putting those simulations into the hands of surgeons.
In research published this week in the journal Nature Biomedical Engineering, Trayanova’s team created personalized heart simulations to guide the surgeries of 10 patients with persistent irregular heartbeats. The virtual hearts predicted where surgeons should destroy heart tissue that could produce erratic electrical signals now and in the future.
The proof-of-concept study sets the stage for an FDA-approved clinical trial of 160 patients slated to begin this fall. That trial will assess whether the virtual heart-guided surgery is more accurate and effective than today’s conventional, one-size-fits-all procedure.
Last year, surgeons used the Johns Hopkins team’s 3D virtual simulation of the heart’s ventricles—the two large, lower chambers—to guide the surgery of five patients with faster-than-normal heartbeats. Trayanova’s team has also used the technology to identify patients at high risk of cardiac arrest.
The current study focuses on abnormal electrical signals in the atria—the two upper chambers of the heart—which cause persistent irregular heartbeats, a common condition known as atrial fibrillation (AF). AF is the most common cause of irregular heartbeats, expected to affect as many as 10 million Americans by 2020. “It’s a huge health care burden,” says Trayanova, director of the Computational Cardiology Laboratory at Hopkins.
Patients can experience either intermittent or persistent AF. In either case, doctors traditionally enter the heart with a catheter and burn away the tissue around the atria’s four pulmonary veins, a region implicated in the electrical misfiring. The procedure works well for patients with intermittent AF, says Trayanova, but not so well for those with persistent AF, particularly when patients have scarring in the tissue, which is age related. These patients commonly return to the operating room for repeat surgeries, even up to four or five times, each time creating more scar tissue in the heart, which can lead to more misfiring.
The new individualized procedure, called Optimal Target Identification via Modeling of Arrhythmogenesis (OPTIMA), could target all the problem areas of the heart in the first surgical attempt, including those that will cause electrical misfiring in the future.
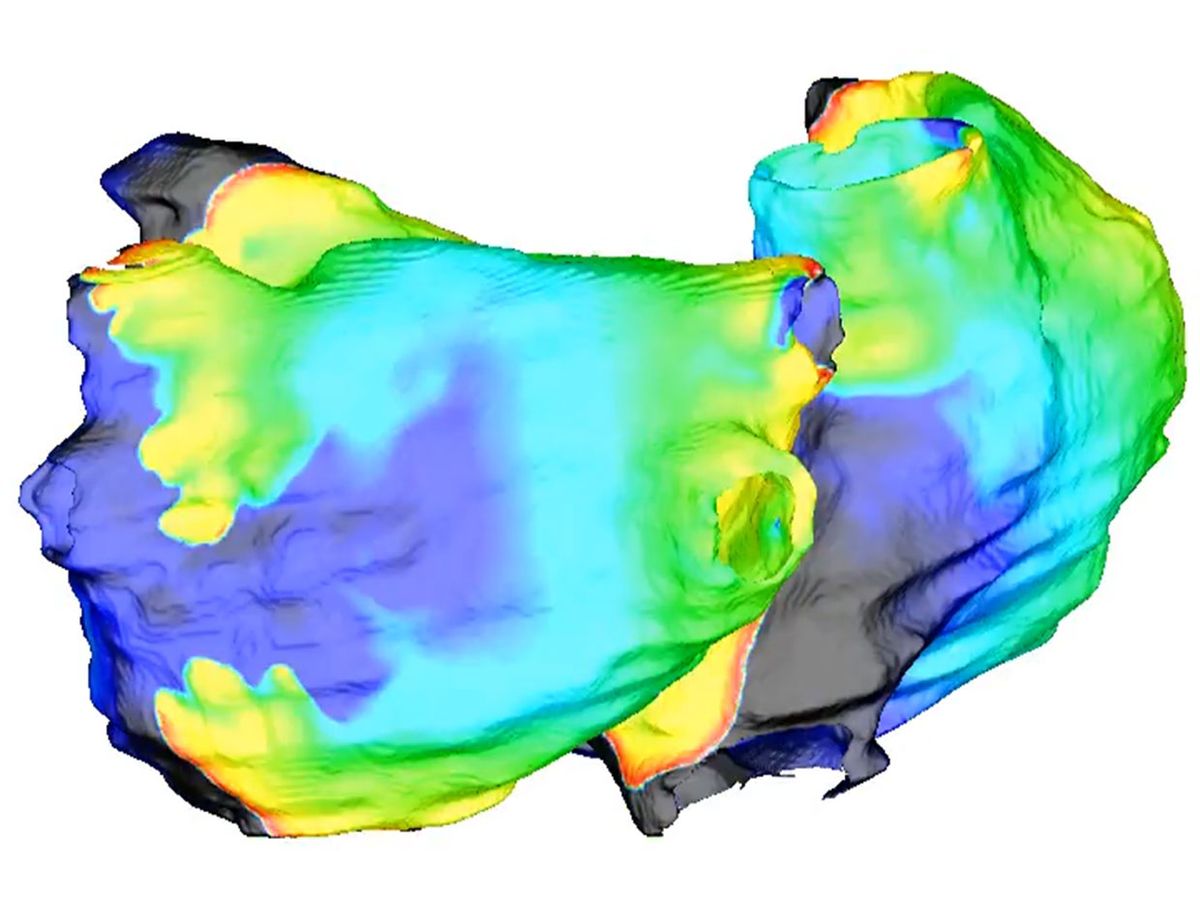
Here’s how it works: First, a patient with AF undergoes contrast-enhanced MRI heart scans, which document any scarring on the heart. Next, the engineers segment the images into a geometric representation of the atria. In a computer program, they populate that representation with virtual heart cells. Cells behave differently around normal or scarred heart tissue, so the digital heart gradually takes on the same behaviors of the patient’s heart.

Next, the engineers prod the virtual heart with small electrical stimuli to see how it will react. “We don’t know a priori, by looking at the image, what’s going to happen,” says Trayanova. So, they watch, and mark down areas where a stimulus prompts an irregular heartbeat. Those are the sites surgeons will need to destroy by burning, a procedure called ablation.
But identifying those initial sites is not enough, says Trayanova. After marking down all the initial problem areas, the team performs virtual surgery, destroying those areas and adding new virtual scars, called lesions, to the model. Then, they do the testing all over again, as new scars can lead to the generation of additional misfiring heart tissue.
“We repeat this several times to find the optimal set of lesions so that when you execute them, the patient never returns to the hospital,” says Trayanova. Typically, by the third round, there are no more hidden areas that can cause abnormal electrical signals.
Finally, an electronic map of the patient’s heart is sent to the system that operating physicians use during surgery, displayed on screens in the operating room. The surgeon uses this map to guide the catheter to the tissues that need to be destroyed.
Normally, an estimated 50 percent of persistent AF patients return to the hospital for additional surgeries. Of the 10 patients who underwent the personalized OPTIMA procedure, only one came back for an additional treatment.
The personalized approach is likely to be more expensive than a typical AF surgery because it involves MRI scanning. However, preventing a patient from returning to the hospital for additional procedures can result in an overall cost savings, says Trayanova. She has submitted a patent application for the technology. “We want a treatment that works, so someone doesn’t have to come in to be ablated five times,” she says.
Megan is an award-winning freelance journalist based in Boston, Massachusetts, specializing in the life sciences and biotechnology. She was previously a health columnist for the Boston Globe and has contributed to Newsweek, Scientific American, and Nature, among others. She is the co-author of a college biology textbook, “Biology Now,” published by W.W. Norton. Megan received an M.S. from the Graduate Program in Science Writing at the Massachusetts Institute of Technology, a B.A. at Boston College, and worked as an educator at the Museum of Science, Boston.