As new, more contagious variants of the coronavirus surge across the planet, public health officials are scrambling to increase genetic sequencing of positive samples. Sequencing is crucial in understanding how the virus is changing, and determining whether our brand new vaccines will remain effective, officials say.
“Imagine if we didn't have this genetic data," says Richard Neher, a professor at the University of Basel who studies the genetic evolution of viruses. “We would see a surge in cases without having any idea what might have changed."
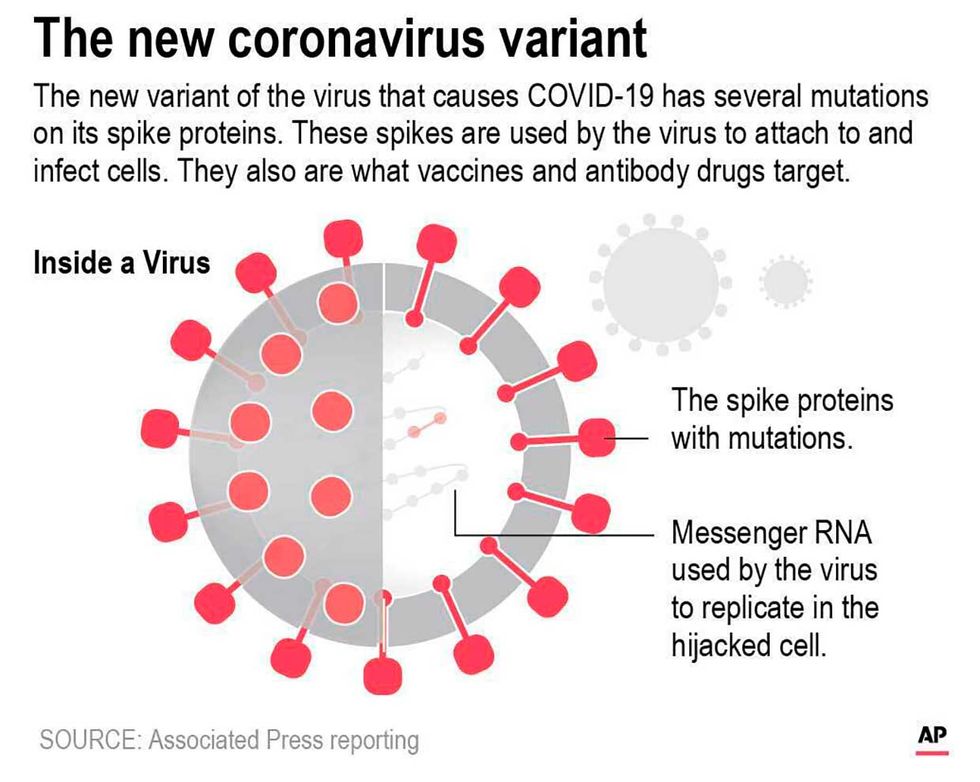
The new variants emerged over the last few months, one out of the United Kingdom and the other out of South Africa. The variant that arose in the UK, dubbed B.1.1.7, correlated with a huge surge in cases there, and has now reportedly been detected in at least 33 countries.
While the variants are likely more contagious, there is no evidence suggesting that they are more deadly or cause more severe disease. Many experts also say that the COVID-19 vaccines that have already been developed will still be effective against the new variants. Still, global surveillance of the virus's genetic sequence is needed to stay on top of the virus's continual adaptations, and plan a vaccine response.
What are viral variants?
Viruses, including SARS-CoV-2 (the coronavirus that causes COVID-19), are constantly mutating. As they move from person to person, their genetic code changes slightly. Most of these mutations are inconsequential, producing no meaningful changes to the structure or function of the virus.
As a virus moves through populations of people, it begins to accumulate enough mutations to lead researchers to call it a “variant" and give it a name. Many variants of the coronavirus have already been recognized.
Sometimes multiple mutations occur quickly, as was the case with the B.1.1.7 variant in the UK. Neher, who helps track the genetic changes in viruses using the software tool Nextstrain, estimates that B.1.1.7 is about 30-35 mutations away from the original strain detected in Wuhan, China at the beginning of the pandemic. Between 10 and 17 of those mutations appeared suddenly, compared with the virus's most recent ancestor.
Many of B.1.1.7's mutations occur in areas of the genome that code for elements of the virus's spike protein. That's important, because the virus's spike protein is what it uses to enter human cells. It's also what our immune systems will recognize when attacking the virus.
Will approved vaccines work against the new variants?
The more the spike protein changes, the harder it is for the immune systems of people who have been vaccinated to mount a swift attack. The same goes for people who have already had COVID-19—their immune systems know the spike protein of the older variants.
But it takes a lot of genetic changes to the spike protein before it can evade our complex immune systems. “The spike protein is a large protein," says Neher. “It's not like a single mutation there would change the virus in a way that it can re-infect everybody on the planet. It's a more of gradual process where some mutations might reduce efficacy of the immune response in some fraction of the population."
The section of the genome that codes for the spike protein is about 3,800 nucleotides, or units, long. So even with a dozen mutations, “for all intents and purposes, it's the same protein," says Neher.
Many experts, including those at the U.S. Centers for Disease Control and Prevention (CDC) and the U.S. National institute of Allergy and Infectious Diseases, have stated publicly that our current vaccines will most likely be effective against the latest variants.
The mutations are “unlikely to have a large impact on vaccine-induced immunity or on existing immunity" from previous infection, said Greg Armstrong, director of the advanced molecular detection program at the CDC, in a media briefing last week.
If the variants do start to evade immune systems of vaccinated people, vaccines can be altered to mimic the new variants. mRNA-based vaccines, such as those developed by Moderna and Pfizer/BioNTech, can be adjusted relatively quickly.
Public health experts push for more genetic sequencing
But to be sure, the public health community will have to keep a close eye on the variants, as well as the virus's future adaptions, which will undoubtedly occur. To that end, experts are calling for larger, more coordinated genetic sequencing and epidemiological surveillance.
In a statement posted December 31, the World Health Organization (WHO) advised the world to “increase routine systematic sequencing of SARS-CoV-2 viruses to better understand SARS-CoV-2 transmission and to monitor for the emergence of variants."
That type of work is not new. Since the earliest weeks of the pandemic, scientists and public health experts globally have been sequencing positive samples and uploading them into public databases such as GISAID. More than 326,000 genomes have been submitted to that database alone—an unprecedented effort, compared with other pathogens.
Still, more is needed. On December 29, the CDC said that the U.S. had about 51,000 sequences in its public databases, noting that the UK had more than twice that many. The CDC now aims to scale up to 3,500 whole genome sequences per week, according to Armstrong at the CDC.
To do that, the agency in November launched the National SARS-CoV-2 Strain Surveillance (NS3) program, and asked each U.S. state to send at least ten samples biweekly for sequencing. The agency is also funding and working with national reference labs and local academic centers to increase sequencing.
Armstrong's division has also been working since 2014 to integrate next-generation sequencing and bioinformatics expertise into state and local health departments. It increased funding for that in December. The effort includes training people to use portable, desktop genetic sequencers such as Oxford Nanopore's MinION and Illumina's MiniSeq.
Emily Waltz is a features editor at Spectrum covering power and energy. Prior to joining the staff in January 2024, Emily spent 18 years as a freelance journalist covering biotechnology, primarily for the Nature research journals and Spectrum. Her work has also appeared in Scientific American, Discover, Outside, and the New York Times. Emily has a master's degree from Columbia University Graduate School of Journalism and an undergraduate degree from Vanderbilt University. With every word she writes, Emily strives to say something true and useful. She posts on Twitter/X @EmWaltz and her portfolio can be found on her website.