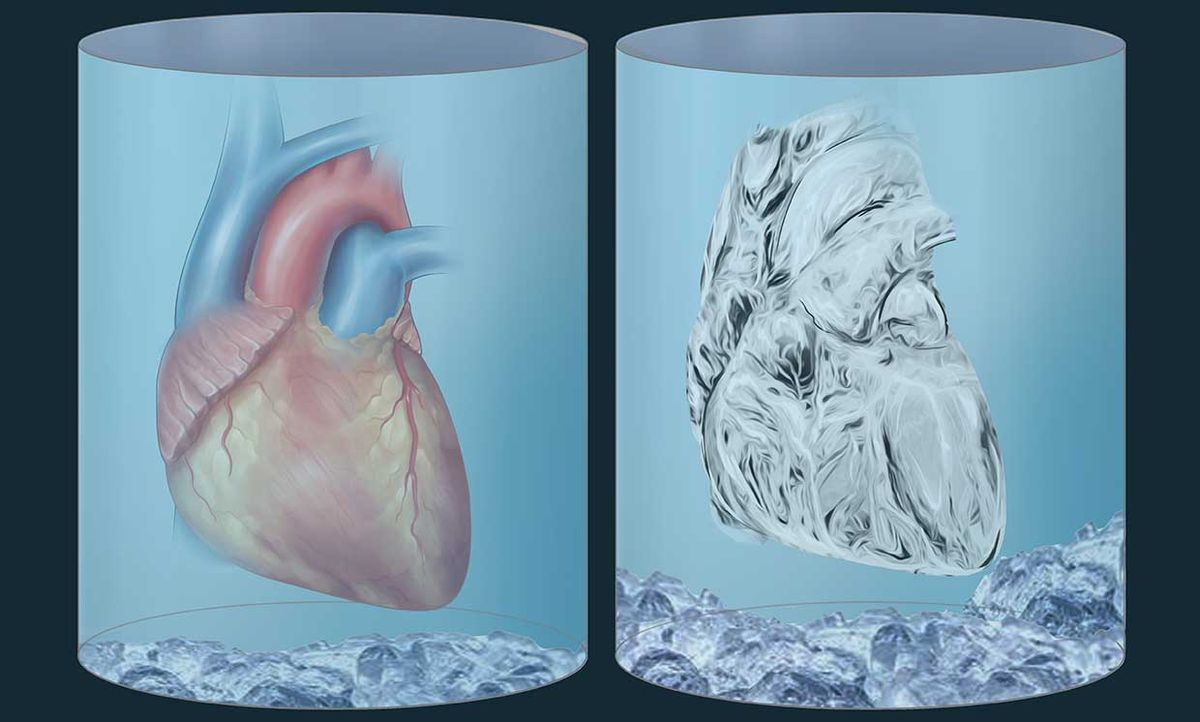
When scientists in the 1950s tried to cool hamsters to 0 °C and rewarm them, it didn’t go great. In 2002, things looked rosier when a scientist chilled a single rabbit kidney down to -130°C, then rewarmed it and transplanted it into a live rabbit, where it worked for 48 days.
Then not much else succeeded for the next decade. Chilling and re-warming organs, it turns out, is really, really hard. Ice crystals that form during cooling and re-warming cause massive physical damage to cells and whole tissues.
To nudge the field forward, in 2014 a group of entrepreneurs from Silicon Valley formed the nonprofit Organ Preservation Alliance (OPA). In the years since, the OPA coordinated numerous conferences and publications and prompted the creation of a National Science Foundation technology roadmap for organ cryopreservation, all of which bolstered more than $100 million in new cryobiology funding from U.S. science agencies, donors, and industry.
Now, in what OPA sees as a culmination of their efforts, the organization is launching a philanthropic fund to support two academic research centers focused on cryopreservation.
“It’s been a renaissance for the field of cryobiology,” says biomedical engineer John Bischof, who will lead one of the centers at the University of Minnesota. The second will be hosted at Massachusetts General Hospital (MGH). “These two centers build on that momentum, and new technologies have come into sharp focus within the same timeframe,” he says.
OPA’s newly-formed Biostasis Research Institute will fund the centers in installments—some of which is being matched by the universities—for about US $3.3 million, says Jedd Lewis, CEO of OPA and co-founder of BRI, along with serial biotech founder Sebastian Giwa. In addition to the BRI funds, last summer Bischof and Mehmet Toner at MGH, along with collaborators at UC Berkeley, UC Riverside, Carnegie Mellon and with help from OPA, secured a 5-year renewable $26 million NSF Engineering Research Center grant to support cryopreservation work.
The BRI centers are tasked with three goals intended to have immediate medical impact: storage of neonatal organs for children in need; long-term storage of brain tissue (no, not for Walt Disney’s brain, but rare, donated brain tissues for research); and extending the storage time of kidneys for transplantation from hours to days and weeks.
The three goals are “tractable,” says Lewis. “We’re trying to help give a push and be a catalyst to get these projects started, until they have self-sustaining momentum.”
At the new Organ Banking Center at MGH, Mehmet Toner, Korkut Uygun and Shannon Tessier are developing tools to safely chill tissues. This involves exploring sub-freezing temperatures, such as by storing human livers at –4 °C to extend the life of the organ by 27 hours, and developing cryoprotectant solutions to be infused into tissues to supplant water and help prevent ice damage.

At the Center for Organ and Tissue Cryopreservation at the University of Minnesota, Bischof, along with transplant surgeon Erik Finger and colleagues, will focus on safely cooling tissues and organs by infusing the vasculature and surroundings with cryoprotective solutions and iron oxide nanoparticles coated with silica. After rapid cooling to a very low temperature, a process called vitrification, the organ or tissue is stored. Upon rewarming, Bischof’s team activates the distributed nanoparticles via a radiofrequency field, heating the cryopreserved tissue rapidly and uniformly. Finally, the cryoprotective agents and nanoparticles can be safely removed prior to transplant or other biomedical use.
With industry collaborators, the Minnesota team is currently installing a large radiofrequency (RF) coil that can activate nanoparticles throughout a one-liter system—about the size one would need for human organs. The Minnesota group is also developing a way scale up nanoparticle production, since the process will require several grams for each preserved organ.
Bischof, who has studied cryobiology his entire career, says scientists are coming back to ideas they had in the 1980s, but now with the right technology to carry them out. “It’s incredibly exciting,” says Bischof. “Back then, we didn’t have the right tools, but now it feels like we really do. We hope we’re about to make this really big step forward.”
Beyond organs, Bischof’s team is also using laser-excited gold nanoparticles to cryopreserve zebrafish and fruit fly embryos used as biomedical model organisms, as well as coral and other aquatic systems for ecosystem restoration.
And as the technology advances, some researchers are advocating for a repository for animal reproductive cells, similar to the Svalbard Global Seed Vault. But instead of remote Norway, this “fauna ark” could be put on the far side of the moon, says Bischof. “If you put it in the right place, it’ll never see sun. It’ll always be in a cryogenic temperature, and it’ll be as stable as one could possibly imagine.”
Megan is an award-winning freelance journalist based in Boston, Massachusetts, specializing in the life sciences and biotechnology. She was previously a health columnist for the Boston Globe and has contributed to Newsweek, Scientific American, and Nature, among others. She is the co-author of a college biology textbook, “Biology Now,” published by W.W. Norton. Megan received an M.S. from the Graduate Program in Science Writing at the Massachusetts Institute of Technology, a B.A. at Boston College, and worked as an educator at the Museum of Science, Boston.