Supercomputer-aided development of possible antiviral therapies, rapid lab testing of other prospective treatments, and grants to develop new vaccine technologies: Coronavirus responses such as these may still be long shots when it comes to quickly containing the pandemic. But these long shots represent one possible hope for ultimately turning the tide and stopping the virus’s rapid development and spread,. (Another possible hope emerges from recent case reports out of China suggesting that many of the worst COVID-19 cases feature a hyperactive immune response called a “cytokine storm”—a physical phenomenon for which reliable tests and some effective therapies are currently available.)
As part of the direct attack on SARS-CoV-2—the virus that causes COVID-19—virtual and real-world tests of potential therapies represent a technology-focused angle for drug and vaccine research. And unlike traditional pharmaceutical R&D, where clinical trial timetables mean that drugs can take as long as 10 years to reach the marketplace, these accelerated efforts could yield results within about a year or so.
For instance, new supercomputer simulations have revealed a list of 77 potential so-called repurposed drugs targeted at this strain of coronavirus.
Researchers say the results are preliminary, and that they expect most initial findings will ultimately not work in lab tests. But they’re reaching the lab testing stage now because supercomputers have made it possible to sort through leagues of candidate therapies in days using simulations that previously took weeks or months.
Says Jeremy Smith, professor of biochemistry and cellular and molecular biology at the University of Tennessee, Knoxville, the genetic sequencing of the new coronavirus provided the clue that he and fellow researcher Micholas Dean Smith (no relation) used to move ahead in their research.
“They found it was related to the SARS virus,” Smith says. “It probably evolved from it. It’s a close cousin. It’s like a younger brother or sister.”
And since the proteins that SARS makes have been well studied, researchers had a veritable recipe book of very likely SARS-CoV-2 proteins that might be targets for potential drugs to destroy or disable.
“We know what makes up all the proteins now,” says Smith. “So you try and find drugs to stop the proteins from doing what the virus wants them to do.”
Smith said working with a short timetable of months not years means limiting the therapies available to be tested. There are, for starters, thousands of molecules naturally occurring in plants and microbes and have been part of human diets for many years. Meanwhile, other molecules have already been developed by pharmaceutical companies for other drugs for other conditions.
“Many of them are already approved by the regulatory agencies, such as the FDA in the U.S., which means their safety has already been tested—for another disease,” Smith says. “It should be much quicker to get the approval to use it on lots of people. That’s the first stage. If that doesn’t work, then we’d have to go and design a new one. Then you’d have your 10-15 years and $1 billion dollar of investment on average. Hopefully, that’d be shortened in this case. But you don’t know.”
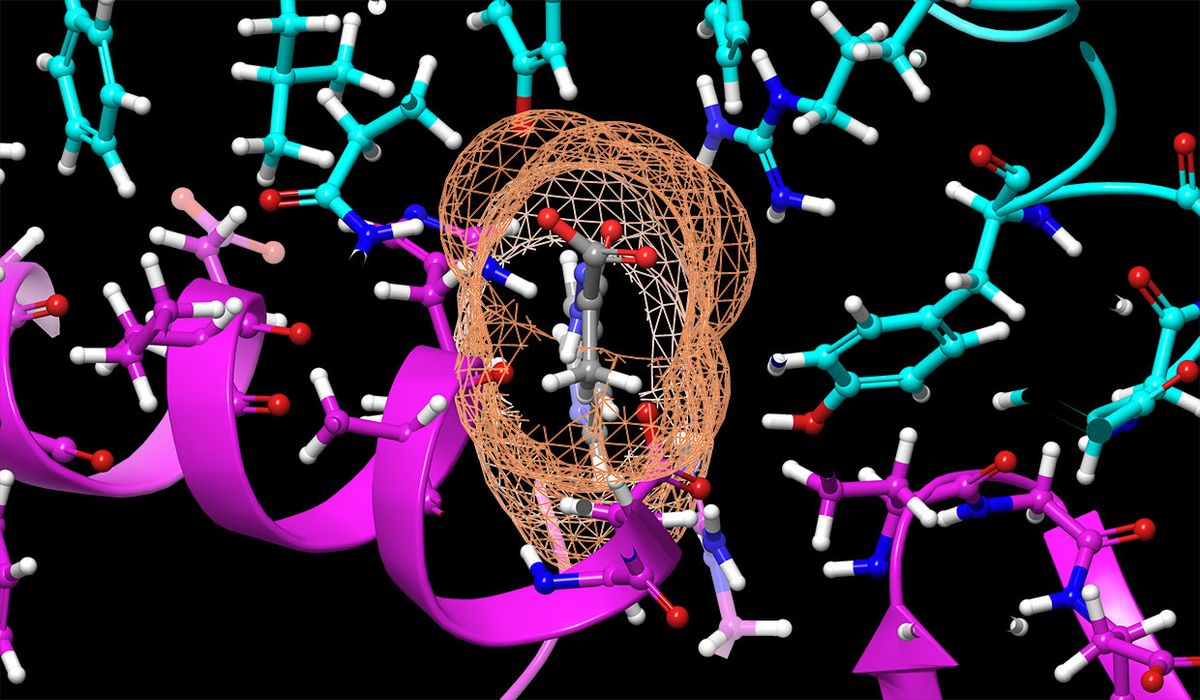
According to Smith, the reason SARS-CoV-2 is called a coronavirus is because of the protein spikes on the outside of the virus make it look like the sun’s corona. Here is where the supercomputing came in.
Using Oak Ridge National Laboratory’s Summit supercomputer (currently ranked the world’s fastest at 0.2 peak exaflops), the two co-authors ran detailed simulations of the spikes in the presence of 9000 different compounds that could potentially be repurposed as COVID-19 drugs.
They ultimately ranked 8000 of those compounds from best to worst in terms of gumming up the coronavirus’s spikes—which would, ideally, stop it from infecting other cells.
Running this molecular dynamics sequence on standard computers might take a month. But on Summit, the computation took a day, Smith recalls.
He said they’re now working with the University of Tennessee Health Sciences Center in Memphis and as well as possibly other wet-lab partners to test some of the top-performing compounds from their simulations on real-world SARS-CoV-2 virus particles.
And because the supercomputer simulation can run so quickly, Smith said they’re considering taking the next step of the process.
“There’s some communication [with the wet lab]: ‘This compound works, this one doesn’t,’” he said. “‘We have a partial response from this one, a good response from that.’ And you would even use things like artificial intelligence at some point to correlate properties of compounds that are having effects.”
How soon before the scientists could take this next step?
“It could be next week, next month or next year,” he said, “Depending on the results.”
On another front, the Bill and Melinda Gates Foundation has underwritten both an “accelerator” fund for promising new COVID-19 treatments as well as an injector device for a SARS-CoV-2 vaccine scheduled to begin clinical trials next month. (The company developing the vaccine—Innovio Pharmaceuticals in Plymouth Meeting, Penn.—has announced an ambitious timetable in which it expects to see “one million doses” by the end of this year.)
One of the first announced funded projects by the “accelerator” (which has been co-funded by Wellcome in the U.K. and Mastercard) is a wet-lab test conducted by the Rega Institute for Medical Research in Leuven, Belgium.
Unlike the Summit supercomputer research, the Rega effort involves rapid chemical testing of 15,000 antiviral compounds (from other approved antiviral therapies) on the SARS-CoV-2 virus.
An official from the Gates Foundation, contacted by IEEE Spectrum, said they could not currently provide any further information about the research or the accelerator program; we were referred instead to a blog post by the Foundation’s CEO Mark Suzman.
“We’re optimistic about the progress that will be made with this new approach,” Suzman wrote. “Because we’ve seen what can come of similar co-operation and coordination in other parts of our work to combat epidemics.”
Margo Anderson is the news manager at IEEE Spectrum. She has a bachelor’s degree in physics and a master’s degree in astrophysics.