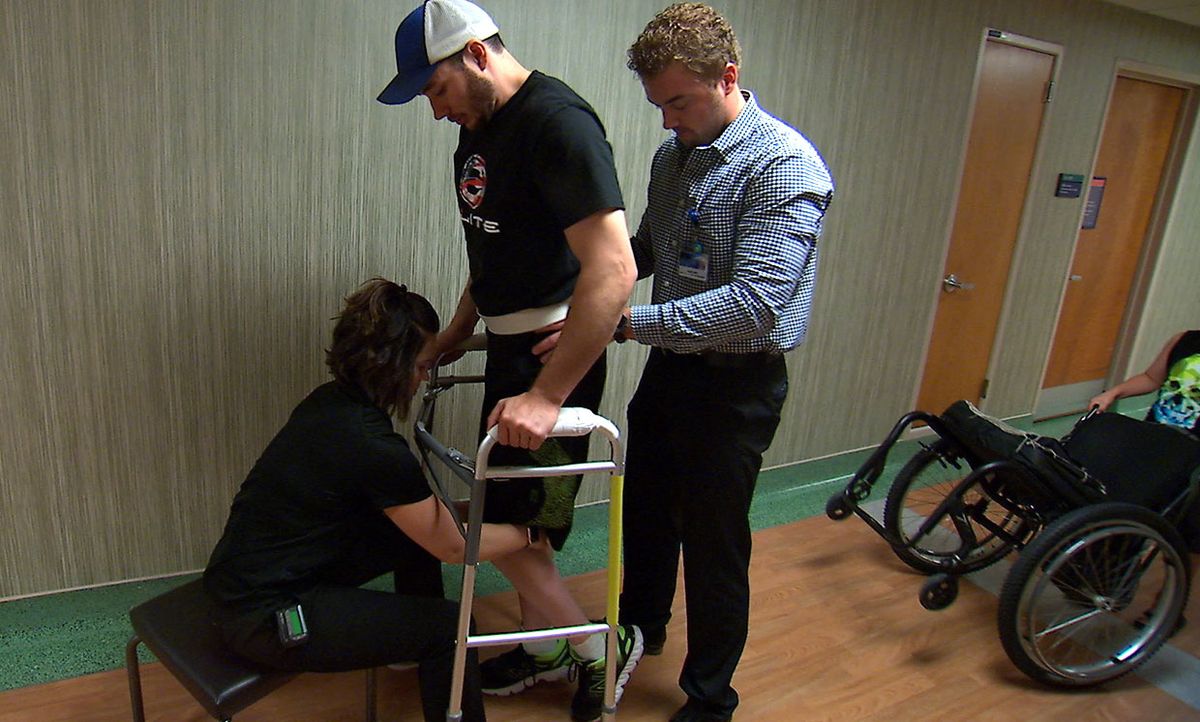
The man’s steps aren’t graceful. His knees are jerky, and his toes often drag. But considering that his legs are paralyzed—and have been since a snowmobile accident four years ago—the fact that he can voluntarily put one foot in front of the other signifies a great stride in the effort to overcome paralysis.
This accomplishment, reported today in the journal Nature Medicine, is the product of high-tech engineering and human grit. The test subject first had an electric gadget implanted in his lower back to stimulate the nerves in his spinal cord. Then he started a rehab regimen, and kept showing up for 43 weeks. In each session, the researchers turned on the stimulator and helped him move his legs. As the weeks went on, he needed less and less help. Eventually he could take steps on his own, using only a walker to stabilize his body.
Finally, in one session toward the end of the study, he walked the length of a football field.
“The patient was able to regain voluntary control of his legs,” said neurosurgeon Kendall Lee, one of the principal investigators, in a press conference. Lee directs the Mayo Clinic’s Neural Engineering Lab. “The patient’s own mind and thoughts were able to drive movement in the legs. We were able to get him to stand independently, and be able to take his own steps.”
When the spinal stimulator is turned off, the patient’s legs are still paralyzed—and he can’t yet use it for his everyday activities. But the gadget the researchers implanted in his back is an FDA-approved neurostimulator currently used for patients with chronic pain. If further studies prove that this treatment is safe and effective for people with spinal cord injuries, many more paraplegic people may soon be rising to their feet.
The results reported today are the culmination of years of research by doctors, biomedical engineers, and physical therapists. In 2013, IEEE Spectrum reported on a precursor study led by Susan Harkema at the University of Louisville that used implanted electrodes to enable paraplegic people to stand independently. One year later, we reported that Harkema’s subjects were beginning to move their paralyzed legs and feet.

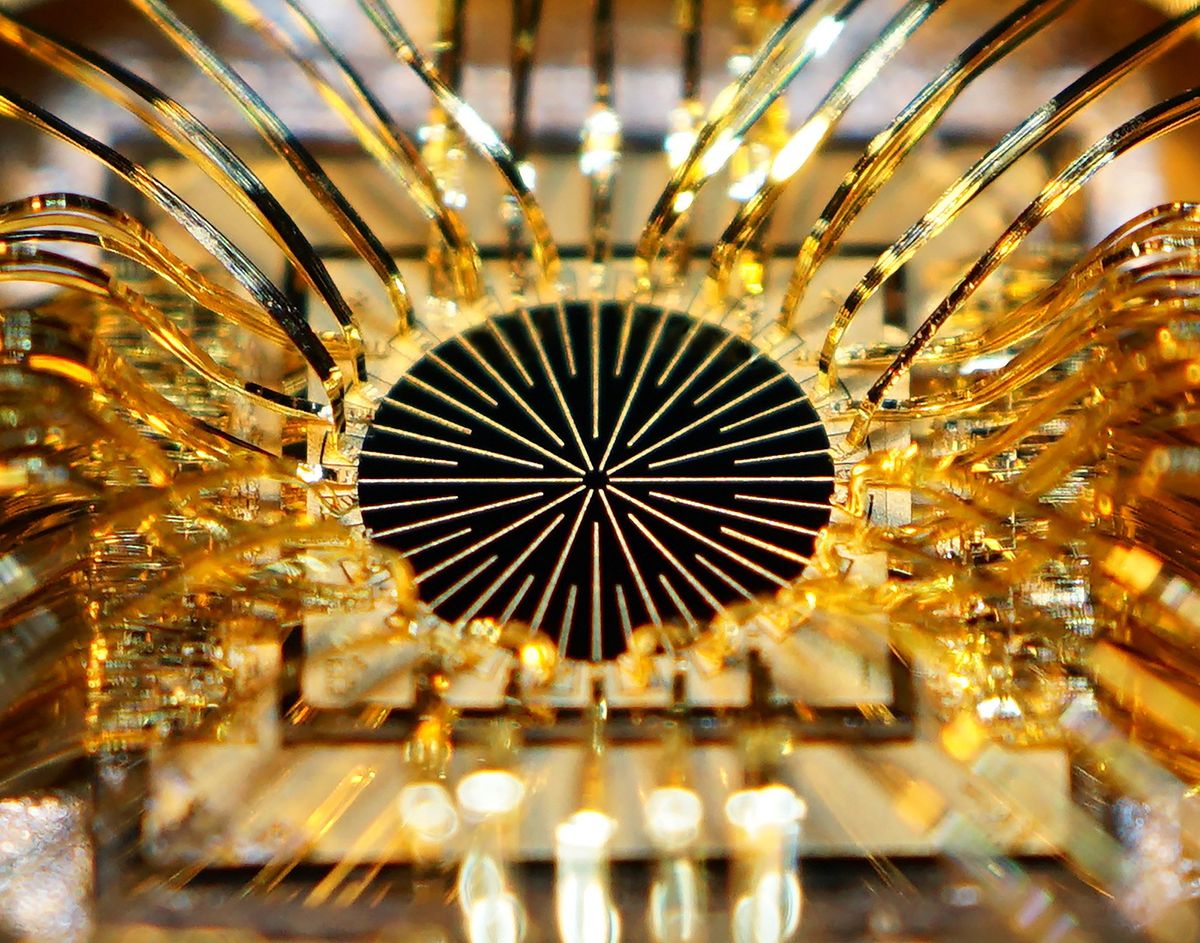
The Mayo Clinic’s star patient had a panel of electrodes placed in the epidural space that surrounds his spinal cord, below the site of his injury and adjacent to the nerves that control the leg muscles. A pulse generator and a battery were implanted elsewhere in his torso. To enable the patient to stand and walk, the researchers didn’t just blast current through the electrodes. Instead they found precise stimulation parameters (including which electrodes were used, the amount of voltage, and the length of each stimulation pulse) that worked for the specific functions of standing and walking.
The rehab was intense. After the patient recovered from surgery, he started that 43-week regimen of physical therapy in which he worked on standing, taking steps on a treadmill, and taking steps on the ground with a walker. The stimulation didn’t restore any sensation to his legs, so he had to watch his legs to see if they were moving as he intended.
So how does it work? How did that stimulation and rehab enable the patient to stand and walk? Surprisingly, the researchers still don’t know.
In the press briefing, Lee and his co-investigator, Mayo Clinic biomedical engineer Kristin Zhao, started to sound a little frustrated as reporters asked repeatedly for an explanation. “We would like to know as well the exact mechanism,” said Lee. “This is very exciting, but it’s still very early in the research stage.”
They’re sure that the stimulation did not cure the patient’s spinal cord injury. It did not cause damaged nerves to regrow.
In an email to IEEE Spectrum, Zhao explains one hypothesis. Research has shown, she says, that even people whose spinal cord injuries have produced “complete loss of function” may still have intact nerve fibers spanning the injury site. “In cases where there is tissue intact, the nerves that send signals between the brain and the spinal cord may not be robust enough to produce functional activity” in normal conditions, she writes. But “epidural stimulation may be enhancing spinal network activity to re-functionalize spared connections.”
The researchers will keep working to understand how they got their remarkable results, and will also experiment with the rehab regimen. There might be ways to shorten and streamline the rehab, Zhao said in the press briefing. They’re also considering whether their approach can be combined with robotic exoskeletons that are now available for people with spinal cord injuries.
The Mayo Clinic study should “give hope to people who are faced with paralysis,” Lee said in the briefing. It’s the latest step toward showing that paralysis need not be a permanent condition.
Eliza Strickland is a senior editor at IEEE Spectrum, where she covers AI, biomedical engineering, and other topics. She holds a master’s degree in journalism from Columbia University.