The Charge of the Ultra-Capacitors
Nanotechnology takes energy storage beyond batteries

In 1995, a small fleet of innovative electric buses began running along 15-minute routes through a park at the northern end of Moscow. A decade later, a few dozen seaport cranes in Asia, a couple of light-rail trains in Europe, and a battalion of garbage trucks in the United States have joined their high-tech ranks.
A smattering of mass-transit vehicles and industrial machines may seem like one wimpy revolution, but revolutionary they are. Unlike most of their electric relatives, these vehicles all share one key attribute: they don't run on batteries. Instead, they are powered by ultracapacitors, which are souped-up versions of that tried-and-true workhorse of electrical engineering, the capacitor.
A bank of ultracapacitors releases a burst of energy to help a crane heave its load aloft; they then capture energy released during the descent to recharge. Buses, trams, and garbage trucks powered by the devices all run for short stretches before stopping, and it's during braking that the ultracapacitors can partially recharge themselves from the energy that's normally wasted, giving the vehicles much of the juice they need to get to their next destinations.
Because no chemical reaction is involved, ultracapacitors--also known as supercapacitors and double-layer capacitors--are much more effective at rapid, regenerative energy storage than chemical batteries are. What's more, rechargeable batteries usually degrade within a few thousand charge-discharge cycles. In a given year, a light-rail vehicle might go through as many as 300 000 charging cycles, which is far more than a battery can handle. (Although flywheel energy-storage systems can be used to get around that difficulty, a heavy and complicated transmission system is needed to transfer the energy.)
The synergy between batteries and capacitors--two of the sturdiest and oldest components of electrical engineering--has been growing, to the point where ultracapacitors may soon be almost as indispensable to portable electricity as batteries are now.
Ultracapacitors are already all over the place. Millions of them provide backup power for the memory used in microcomputers and cellphones. They also supply brief bursts of energy to numerous consumer products containing batteries. In a camera, for example, an ultracapacitor can extend battery life by providing the oomph for power-intensive functions, like zooming in for a close-up.
Perhaps most exciting is what ultracapacitors could do for electric cars. They're being explored as replacements for the batteries in hybrid cars. In ordinary cars, they could help level the load on the battery by powering acceleration and recovering energy during braking. Most deadly to the life of a battery are the moments when it is subjected to high-current pulses and charged or discharged too quickly. Conveniently, delivering or accepting power during short-duration events is the ultracapacitor's strongest suit. And because capacitors function well in temperatures as low as –40 C, they can give electric cars a boost in cold weather, when batteries are at their worst.
Commercially available ultracapacitors already address those needs to some extent and can provide many times the power of batteries of the same weight or size. But in terms of the amount of energy they can hold, ultracapacitors lag far behind. The major difference is that batteries store energy in the bulk of their material, whereas all forms of capacitors store energy only on the surface of a material. Like a battery, an ultracapacitor is filled with an ionic solution--an electrolyte--and its current collectors attach to the electrodes and conduct current to and from them. The collectors are coated with a thin film of activated carbon that has orders of magnitude more surface area than ordinary capacitors. The amount of surface area in ultracapacitor designs has so far been constrained by the limitations in the porosity of the activated carbon.
The innovation that my colleagues John Kassakian and Riccardo Signorelli and I have pursued at MIT is to replace the activated carbon with a dense, microscopic forest of carbon nanotubes that is grown directly on the surface of the current collector. We think--and our work so far supports our theory--that by doing so, we can create a device that can hold up to 50 percent as much electrical energy as a comparably sized battery. This feat would allow ultracapacitors to supplant batteries in a number of mainstream applications.
It's almost engineering heresy to suggest that a capacitor could power a car. Indeed, the common capacitor stores a puny amount of energy. At equivalent voltage, a chemical battery can store at least a million times as much energy as a conventional capacitor of the same size. Put two ordinary capacitors the size of a D-cell battery in your flashlight, each charged to 1.5 volts, and the bulb will go out in less than a second, if it lights at all. An ultracapacitor of the same size, however, has a capacitance of about 350 farads and could light the bulb for about 2 minutes.
Before delving into our methods, I should explain the basics of capacitors and ultracapacitors. Capacitors have been around since 1745, beating batteries to the scene by half a century. Ultracapacitors are much more recent, but they're not exactly new, either. Engineers at Standard Oil patented ultracapacitor technology in 1966, an unanticipated product of their fuel-cell research. Standard Oil licensed the technology to NEC Corp., of Tokyo, which commercialized the results as ”supercapacitors” in 1978, to provide backup power for maintaining computer memory.
A capacitor consists of two electrodes, or plates, separated by a thin insulator. When a voltage is applied to the electrodes, an electric field builds up between the plates. A capacitor's energy is stored in such an electric field, without requiring any sort of chemical reaction. Thus a capacitor has an almost unlimited lifetime. It's also fast. Depending on its physical structure, typical charge and discharge times are on the order of a microsecond; sometimes they are as quick as a picosecond.
Three main factors determine how much electrical energy a capacitor can store: the surface area of the electrodes, their distance from each other, and the dielectric constant of the material separating them. However, you can push conventional capacitor designs only so far. What the Standard Oil engineers did was to develop a capacitor that functions differently. They coated two aluminum electrodes with a 100-micrometer-thick layer of carbon. The carbon was first chemically etched to produce many holes that extended through the material, as in a sponge, so that the interior surface area was about 100 000 times as large as the outside. (This process is said to ”activate” the carbon.)
They filled the interior with an electrolyte and used a porous insulator, one similar to paper, to keep the electrodes from shorting out. When a voltage is applied, the ions are attracted to the electrode with the opposite charge, where they cling electrostatically to the pores in the carbon. At the low voltages used in ultracapacitors, carbon is inert and does not react chemically with the ions attached to it. Nor do the ions become oxidized or reduced, as they do at the higher voltages used in an electrolytic cell.
This approach allowed the engineers at Standard Oil to build a multifarad device. At the time, even large capacitors had nowhere near a farad of capacitance. Today, ultracapacitors can store 5 percent as much energy as a modern lithium-ion battery. Ultracapacitors with a capacitance of up to 5000 farads measure about 5 centimeters by 5 cm by 15 cm, which is an amazingly high capacitance relative to its volume. The D-cell battery is also significantly heavier than the equivalently sized capacitor, which weighs about 60 grams.
Hundreds of thousands of ultracapacitors are manufactured each year, for applications that require rapid recharging, high power output, and repetitive cycling. In 2005, the ultracapacitor market was between US $272 million and $400 million, depending on the source, and it's growing, especially in the automotive sector. Though ultracapacitors have generally remained a niche player, the situation may soon change.
My laboratory at MIT--the Laboratory for Electromagnetic and Electronic Systems--works with several automobile manufacturers to investigate ways to improve vehicle performance. About four years ago, I assisted on a project to evaluate commercial ultracapacitors for use in cars. While on a flight from Boston to Detroit, I read an article describing a way to grow vertically aligned carbon nanotubes on a flat surface. This is a truly amazing process. A sheet of silica is covered with a nanometer-thick layer of an iron catalyst. The sheet is placed in a vacuum, heated to 650 C, and exposed to a thin hydrocarbon gas, perhaps ethanol or acetylene. The heat causes the iron to form tiny droplets, which steal carbon molecules from the gas. The carbon molecules then begin to self-assemble into tubes, which grow upward from each of the droplets.
By virtue of their dimensions, it struck me that those nanotubes held the promise of even higher porosity than the activated carbon used in commercial ultracapacitors. Together the nanotubes have an enormous surface area, and their dimensions are more uniform than those of the activated-carbon pores, making them more like a paintbrush than a sponge.
There are two major limitations to the conductivity of activated carbon--the high porosity means there isn't much carbon material to carry current, and the material must be ”glued” to the aluminum current collector using a binder, which exhibits a somewhat high resistance. If my colleagues and I replaced the activated carbon with billions of nanotubes, we predicted we could make an ultracapacitor that could store at least 25 percent--and perhaps as much as 50 percent--of the energy in a chemical battery of equivalent weight. (To get that much improvement, we'd have to make a number of other changes, as well, such as increasing the number of ions in the electrolyte to reflect that new-found storage space.)
Another advantage of nanotubes over activated carbon is that their structure makes them less chemically reactive, so they can operate at a higher voltage. And certain types of nanotubes, depending on their geometry, can be excellent conductors--which means they can supply more power than ultracapacitors outfitted with activated carbon [see illustration, "Piling on The Farads"].
Even better, this nanotube-enhanced ultracapacitor would retain all the advantages ordinary ultracapacitors have over batteries: they would deliver energy in quick bursts, they would perform well in cold weather, and they would have much longer life spans. If this ultracapacitor could be developed, it would be revolutionary.
It was clear from the outset that a lot of know-how would be needed to make an ultracapacitor according to our design--knowledge of chemical-vapor deposition, electron microscopy, material science, quantum chemistry. And it's a challenge to get people with all those skills together. One of the strengths of a research university is its incredible diversity of expertise and equipment, plus there's the willingness of faculty to collaborate. Nobody in my lab had experience fabricating carbon nanotubes, but much of the early research in that area at MIT was done in the building next door, at a laboratory under the direction of Mildred Dresselhaus. Using those facilities and aided by Dresselhaus and her lab colleagues, we succeeded in synthesizing a nanotube forest on a small piece of silica in only a few months.
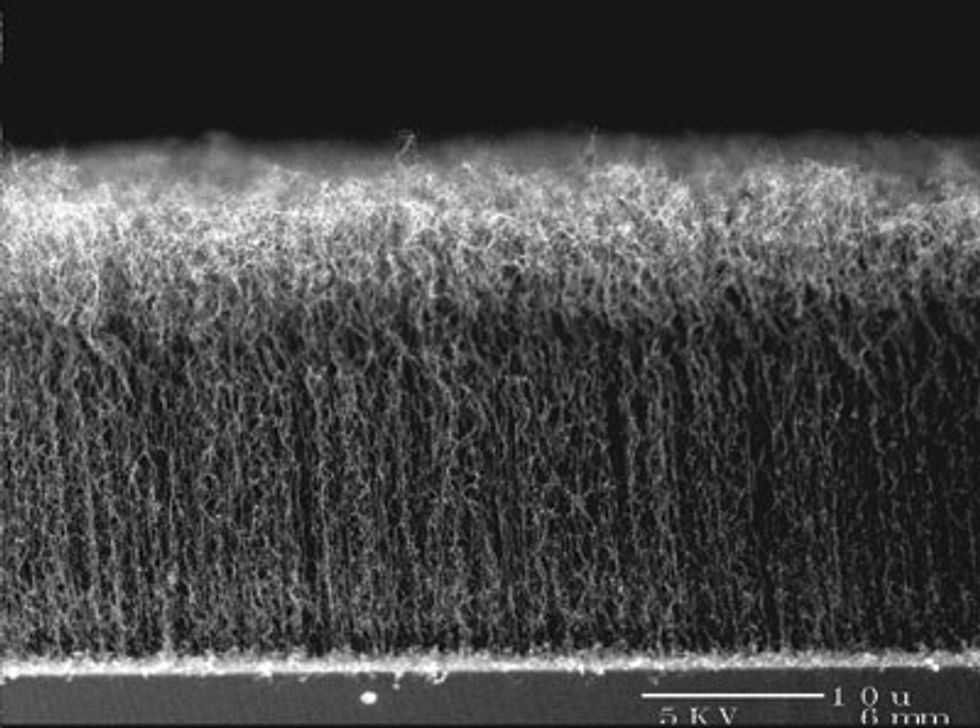
Nanotubes can vary in size, and the ones we're growing are about 5 nm across, or about 1/10 000th the diameter of a human hair. Each tube is about 100 µm long, and they can be spaced as little as 5 nm apart [see image, "Electric Shag," below].
But the sliver of silica was only the start. Silica is an insulator, and we needed a conducting material. After more than a year of false starts, we finally designed and built a custom reactor for chemical-vapor deposition and have used it to grow nanotubes on a conducting substrate. We are now packaging this collection of nanotubes in a prototype ultracapacitor.
We believe that within a few months we'll be able to demonstrate results that outperform today's designs by a wide margin. There will still be a big challenge ahead of us at that point: to see whether our devices can be manufactured at prices that make them attractive for mainstream applications. We are optimistic, though, because chemical-vapor deposition is already used on a huge scale in semiconductor manufacturing, and the raw materials that we need are cheap.
It's not a straight path from high-density ultracapacitors to practical electric cars, but what my colleagues and I have done may constitute one big step along a tortuous route to making such vehicles more convenient and attractive to consumers. Even if it takes many years before ultracapacitors on their own can power either full battery-electric or hybrid cars, we're already at the point where such devices could easily assist lithium-ion batteries [see illustration, "How to Ultracap A Car"]. When the car's electric motor needs high current for a short time, the ultracapacitor supplies it. After the demand eases, the ultracapacitor recharges from the battery. When the motor, working now as a generator, delivers high current for a brief interval--which is typically what happens with regenerative braking--the same thing happens in reverse. A computer would monitor voltages, the state of charge, load, and demand, and then adjust the current flow accordingly using some additional dc-dc power electronics. The added weight and expense involved might not matter if it improves vehicle performance and makes the battery last longer.
Small-cell ultracapacitors can be used in cars for purposes other than in the drivetrain. They can be integrated into air-conditioning, electric power steering, power locks, and window systems--components that demand high peak currents, which typically require large-diameter wiring. The need is intermittent, and the average power is low, so having ultracapacitors provide the high current at strategic points would permit thinner wiring to be installed. With the high price of copper these days, such changes can shave an appreciable amount from the cost of a vehicle.
Safety is another motivation. Suppose a car has electrically actuated brakes or door locks and the wiring harness fails because of a defect or an accident. A local ultracapacitor can still provide power for a few precious seconds or minutes.
Such devices are by no means limited to vehicles. Society is in the midst of an energy crisis, and many sources of green energy would benefit from regenerative energy storage. Electric power grids could be 10 percent more efficient if there could be simple, inexpensive ways to store energy locally at the point of use. And if renewable energy is ever to displace fossil fuels, engineers will need to devise better ways to store wind power when the wind is not blowing and solar power when the sun is not shining.
My colleagues and I are not the only ones researching ultracapacitor technology, of course. All the existing ultracapacitor manufacturers--including Maxwell Technologies, NessCap, Panasonic, Nippon Chemi-Con, and Power Systems Co.--are working on improved activated carbons or devices where one electrode functions as a battery and the other as an ultracapacitor. The Japanese government has provided $25 million for nanotube research, money that has supported a promising joint effort between Nippon Chemi-Con and AIST National Lab to explore nanotube-based techniques. Investigators at Rensselaer Polytechnic Institute, in Troy, N.Y., recently announced, in the Proceedings of the National Academy of Sciences of the United States of America, an exciting combined battery-nanotube ultracapacitor fabric to store electrical energy.
And nanotube forests are not the only way to provide increased porosity. Power Systems, in Japan, for example, has been getting good results with a type of graphene structure that it calls a ”nanogate.”
There's a slightly different approach to modified capacitors that has been generating a lot of buzz lately, developed by a start-up called EEStor, in Cedar Park, Texas. EEStor has focused on improving the dielectric, rather than the capacitor's plates. Its design uses barium titanate, which has a high dielectric constant. High-dielectric-constant substances allow for high-value capacitors that are still small in size. The downside is that such materials generally are unable to withstand electrostatic fields of the same intensity as low-dielectric-constant substances such as air. EEStor claims that the capacitors can operate at extremely high voltages, on the order of several thousand volts, leading to very high storage capacities. One concern is that high voltages can cause a dielectric to break down irreversibly in the presence of even slight imperfections in the material. Only time will tell how its design fares.
Improving substantially on the means to store electrical energy would be a welcome development, and high-density capacitive storage is one promising avenue of research. Although batteries and capacitors are old inventions, our particular technique could not have been pursued until recently. Just as semiconductor designers have created smaller and smaller transistors, so have engineers in other areas learned to manipulate objects with ever-more-minuscule dimensions. The ability to sculpt materials at the atomic level is new and evolving. Engineers can use these new techniques to achieve novel properties and, in the case of my lab's research, to move toward a nanoengineered carbon that might usher in the next generation of energy storage.
About the Author
JOEL SCHINDALL spent 35 years working in the telecommunications and satellite industries before joining the faculty of MIT, where he is now associate director of the Laboratory for Electromagnetic and Electronic Systems.
To Probe Further
For an overview of ultracapacitors and their applications, as well as a number of free technical papers (after registration), visit https://www.maxwell.com/ultracapacitors/technical-support/white_papers.asp.
The National Renewable Energy Laboratory, in Golden, Colo., surveys its energy storage research at https://www.nrel.gov/vehiclesandfuels/energystorage/ultracapacitors.html.