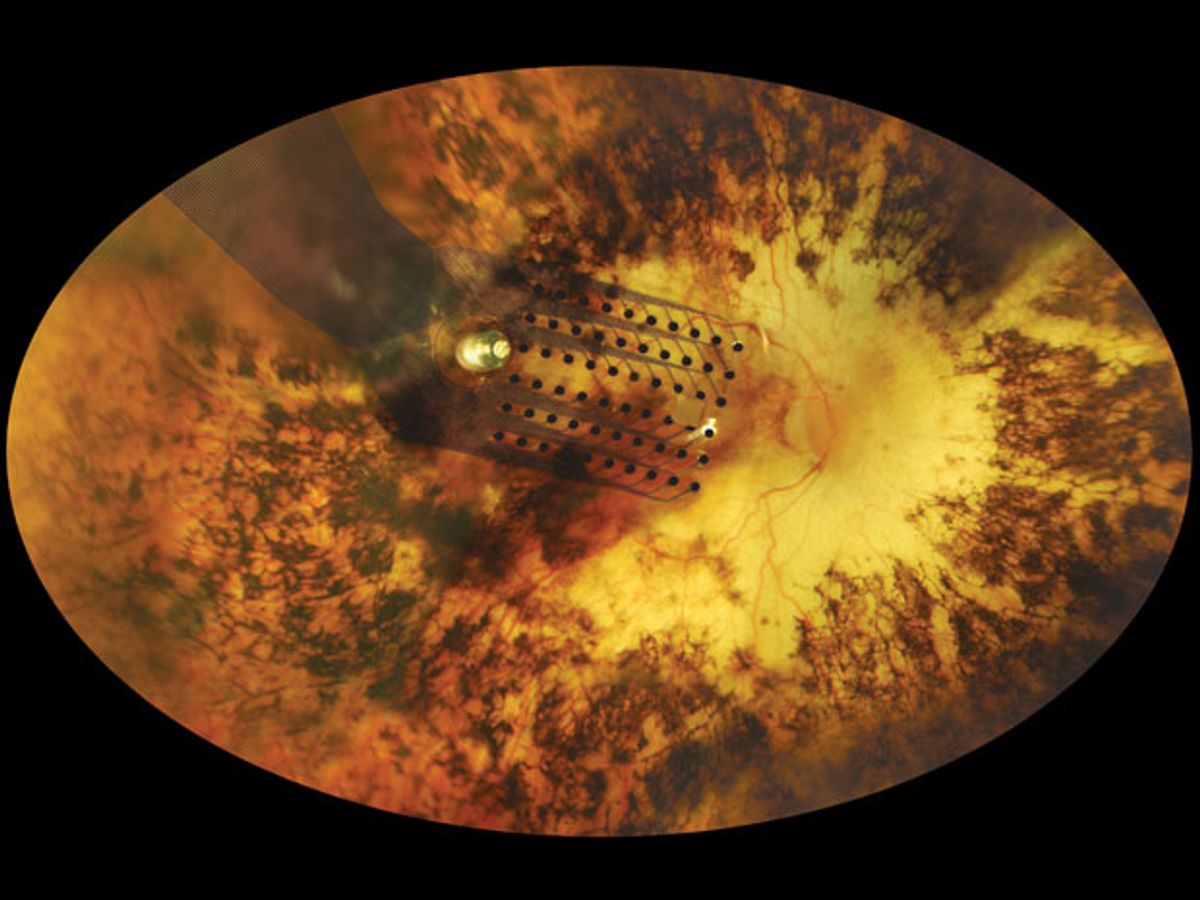
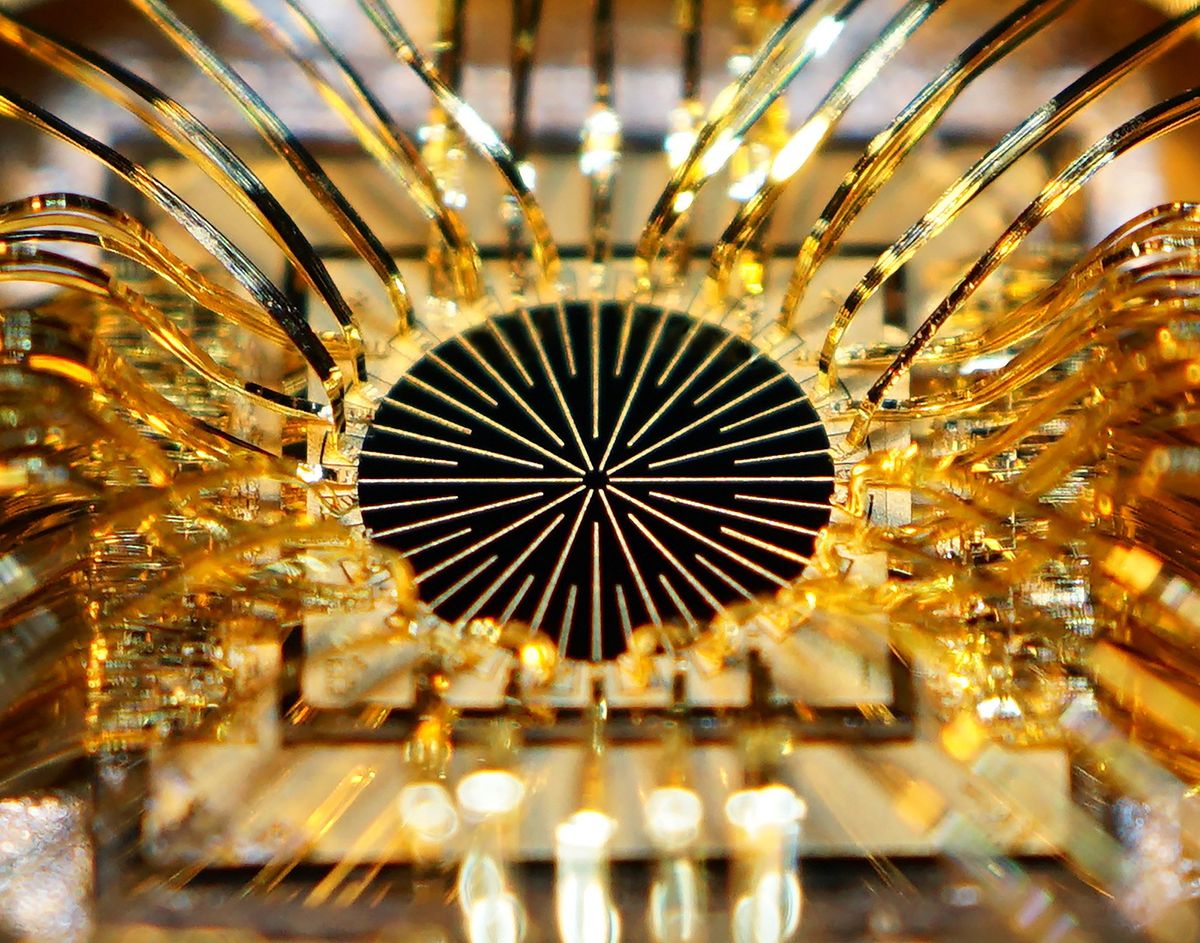
The treatment involves electrodes implanted in the eyes of people whose retinas are damaged. The FDA approved the implants for people with severe cases of retinitis pigmentosa, a relatively small patient population. But the company that makes the implants, Second Sight Medical Products, says they can benefit a much broader group of people with vision problems, including many elderly people who suffer from macular degeneration.
That article was part of our "Top Tech 2012" special report based on Second Sight's optimistic predictions that it would win FDA approval for the implants in the year 2012. So the company is a couple of months behind schedule in the United States, but its implants have been on the market in Europe since 2011.
Second Sight isn't the only company working on retinal prostheses. We've also described a competing technology from the German company Retina Implant AG, whose system was undergoing clinical trials last year.
Photos: Second Sight, David Yellin
Eliza Strickland is a senior editor at IEEE Spectrum, where she covers AI, biomedical engineering, and other topics. She holds a master’s degree in journalism from Columbia University.