Melanoma, the deadliest form of skin cancer, often causes subtle changes to the skin, such as discoloration or slightly enlarged moles. The usual detection method, a visual inspection of the skin, can overlook such signs. Instead of just looking for skin cancer, however, it might be better to sniff for it.
IEEE Member A.T. Charlie Johnson, a physics professor at the University of Pennsylvania, in Philadelphia, and his team have developed a DNA-coated nanosensor that can sense the odor from human skin cells that have turned cancerous. The team’s so-called electronic nose is expected to reach clinical settings within the next two years. As with most cancers, the survival rate for melanoma depends on how early it is detected. According to the World Health Organization, more than 65 000 people die each year from the disease.
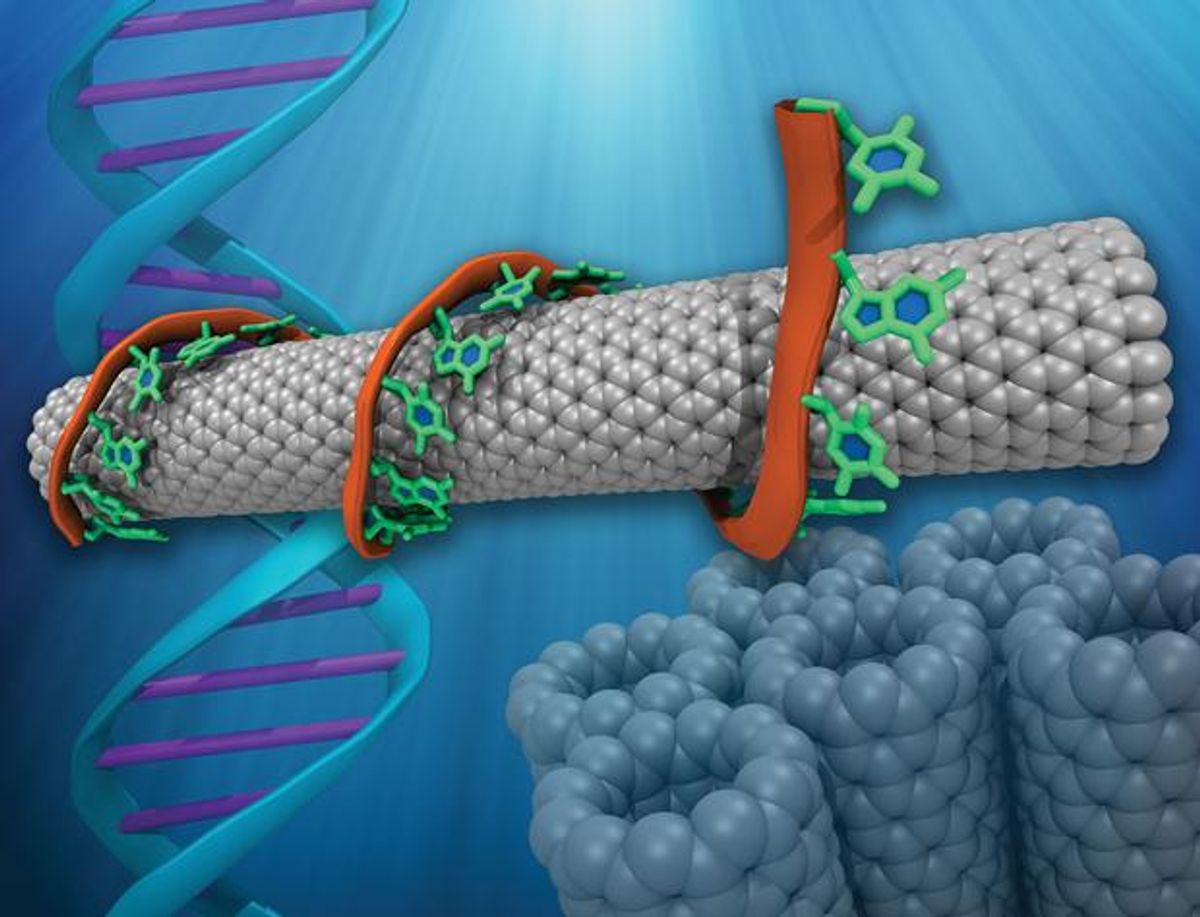
With collaborators at the Monell Center, a research laboratory in Philadelphia focused on the senses of smell and taste, Johnson’s team was able to identify dimethyl sulfone, a volatile organic compound (VOC) specific to melanoma. The compound cannot be perceived by the human olfactory system.
“Our bodies make this compound, but we can’t smell it,” Johnson says. “In contrast, the sensor system we’ve developed using carbon nanotubes can detect dimethyl sulfone from melanoma down to concentrations of a few parts per billion.”
ANIMAL SYSTEM

A growing body of research finds that odor can be used to detect several types of diseases, Johnson notes. The spur to such research has been the canine olfactory system, including well-documented research on dogs’ ability to sense odor associated with lung cancer, he says.
Some breeds have a sense of smell estimated to be at least a million times more sensitive than that of humans. No wonder. The human nose has approximately 5 million scent receptors; a bloodhound’s has about 300 million.
Dog biology provided the blueprint for designing the electronic olfactory system, according to Johnson. His team’s e-nose aims to replicate a dog’s sense of smell with thousands of odor-detecting receptors built into the sensor. The receptors are made with single-strand DNA oligomers, or molecular complexes, coated onto a large array of carbon nanotube transistors. These transistors are then placed inside an instrument that captures the vapor released by skin, Johnson says. The vapor interacts with the DNA strands, leading to changes in the electrical characteristics of the nanotube transistors that can be used to identify a number of VOCs, not just dimethyl sulfone.
The array output will contain information from the thousands of different DNA-based receptors, which will then be combined in a manner similar to how the olfactory cortex in the brain processes messages from the olfactory receptor neurons. In a real-world application, the device could draw vapor from a lesion on the skin suspected of being melanoma. Eventually, it might also be possible to detect cancer by sniffing the VOCs in a patient’s blood, saliva, or urine, according to Johnson.
Adamant Technologies, a San Francisco company that produces chemical sensors for a range of medical applications, is working to bring the e-nose developed by Johnson’s team to clinical settings. It’s important that the rate of false positives—a positive diagnosis of a patient who is free of disease—be very low, Johnson explains. “That’s one of the difficulties of taking a new type of screening method to a mass scale,” he says. “False results could cause more harm than good.”
PERSONALIZED MEDICINE
With the new nanosensor, not only might it be possible to more easily detect early stage melanoma, but the cancer’s progress or decline might also be monitored, and that information could affect how the disease is treated.
“Physicians won’t just have one piece of information about the skin cancer but ideally several pieces of information from a compound that will provide a much more personalized look at treatment options,” Johnson says.
Results from the screenings could lead to cancer therapies specific to individual patients, he says, adding: “Working with scientists in the biomedical field, learning from them, and helping them to understand the potential of nano-enabled devices is going to let our team do many things in the area of diagnosis of disease that we couldn’t do before.
Monica Rozenfeld is a freelance writer and an executive communications strategist for Fortune 100 companies.