In the continuing saga of nanotechnology’s application for fuel cells in this blog (see here and here) I have come to realize that there is a fervent belief among some that the reason we don’t have fuel cells powering our automobiles today is the result of a conspiracy among certain interests (presumably the oil industry) to block their use.
I really try to avoid taking sides in this rather fruitless debate. Instead I merely point out how nanotechnology has sometimes failed or succeeded in its intended applications for fuel cells. In either case, I try to put it into context. The current context by the way is that fuel cells power all sorts of things today, but when it comes to automobiles it is only for prototypes.
So, with this latest research coming out of Oxford University in the UK I am going to avoid pronouncements that it will usher in fuel-cell powered cars, or not.
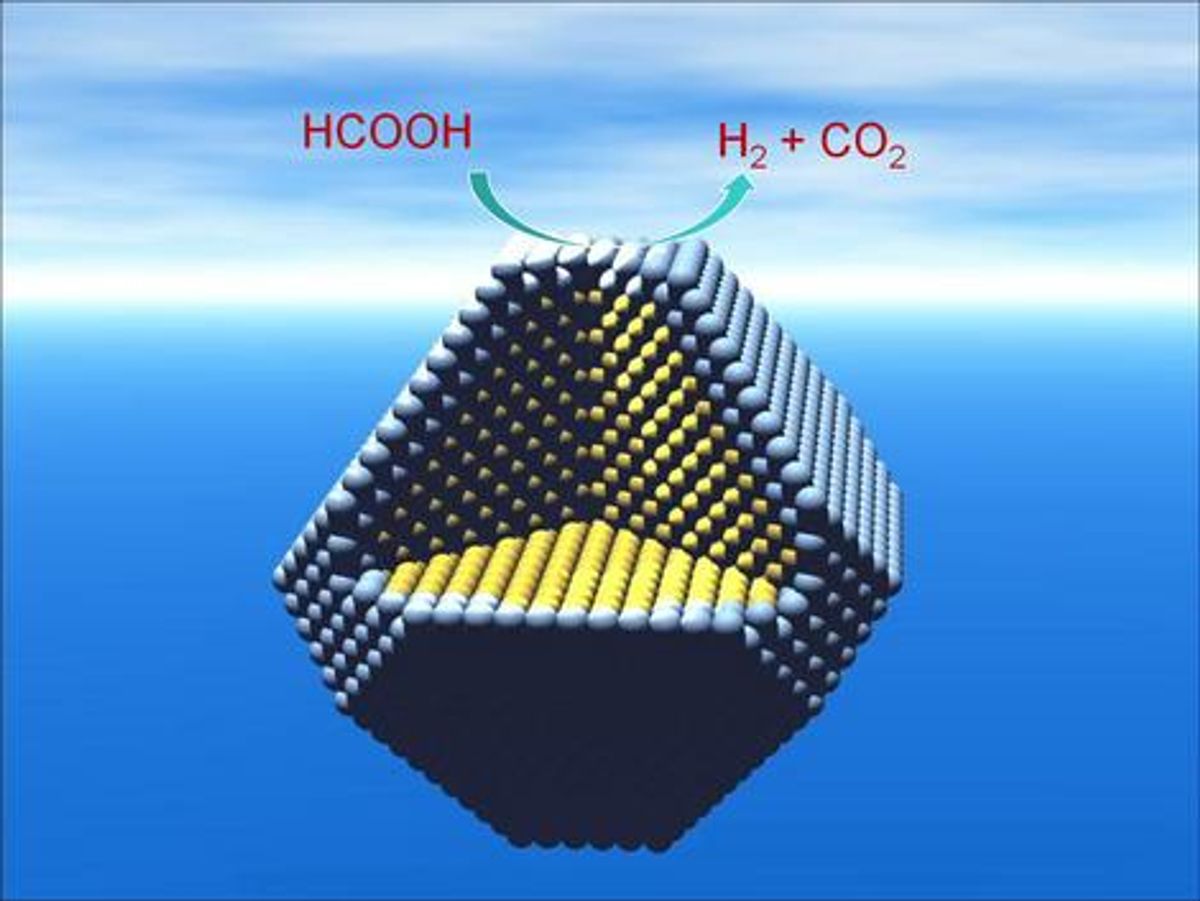
Despite all sorts of interminable calculations to the contrary, hydrogen is flammable and is not as easy or as safe to store as formic acid. As the Nature Nanotechnology abstract explains, “Formic acid (HCOOH) has great potential as an in situ source of hydrogen for fuel cells, because it offers high energy density, is non-toxic and can be safely handled in aqueous solution.”
Edman Tsang of Oxford University’s Department of Chemistry adds in the article reporting on the research “that the storage and handling of organic liquids, such as formic acid, is much easier and safer than storing hydrogen.” (Do I really need to add a video of hydrogen gas exploding?)
The main achievement of the research was the determination that the underlying silver nanoparticles enhance the catalytic properties of palladium. Tsang believes that these improved catalysts could “enable the production of hydrogen from liquid fuel stored in a disposable or recycled cartridge, creating miniature fuel cells to power everything from mobile phones to laptops.” However, I still argue carrying around a container for formic acid in your laptop or cell phone is a bit of a hard sell.Even Tsang concedes, “There are lots of hurdles before you can get a real device, but we are looking at the possibility of using this new technology to replace lithium battery technology with an alternative which has a longer lifespan and has less impact on the environment.”
I’d argue that even if you got all the engineering perfectly sorted, you would still face just some commercial market realities that in the current environment would be hard to overcome.
Dexter Johnson is a contributing editor at IEEE Spectrum, with a focus on nanotechnology.