When it comes to cancer treatment and nanoparticles, the preference has been to use gold nanoparticles. Silicon nanoparticles, on the other hand, have been limited mainly to the realm of electronics applications.
Now researchers at Lomonosov Moscow State University and the Leibniz Institute of Photonic Technology in Germany have demonstrated that silicon nanoparticles can be applied to the diagnosis and treatment of cancer. The researchers claim that this work represents the first time nanoparticles have penetrated into diseased cells and completely dissolved after delivering their cancer treatment drug payload.
The research represents another step in the field of so-called theranostics, in which a diagnostic tool is combined with a therapy tool to treat disease. While many of these theranostic techniques have involved nanoparticles, the problem with metal nanoparticles is that they present biocompatibility issues. Despite delivering drugs accurately to a target such as a group of cancer cells and providing fast action, there are negative side effects such as kidney and liver damage.
“The reason is that gold, silver, titanium oxide, cadmium selenide, and plenty of other nanoparticles are almost not excreted,” said Liubov Osminkina, senior research fellow at Lomonosov Moscow State University, in a press release. “When nanoparticles reach the bloodstream, they can get stuck in internal organs, and after a while they begin to harm the organism due to prolonged toxic effects.”
In their search for biocompatible nanoparticles, the researchers realized that porous silicon was not only biocompatible but could even provide a health benefit. When these silicon nanoparticles dissolve, they become silicic acid, which is a vital substance for strong bones and healthy connective tissues.
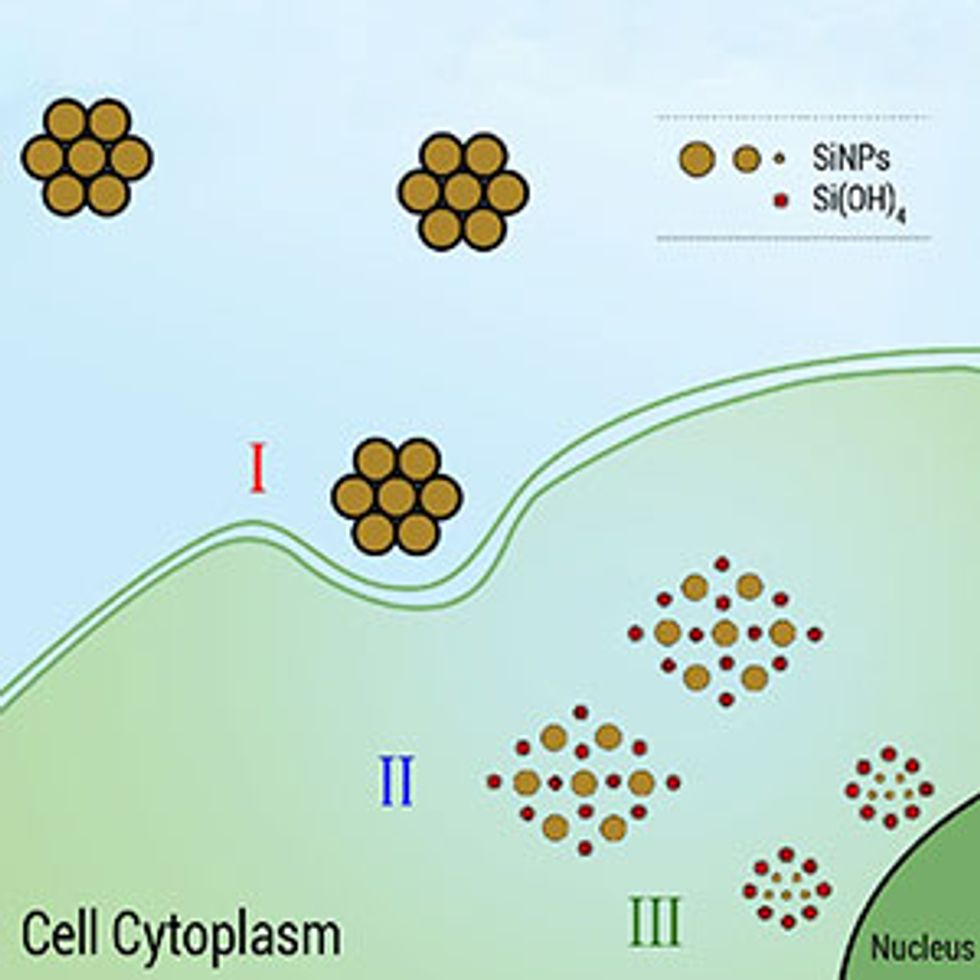
In research described in the journal Nanomedicine: Nanotechnology, Biology and Medicine, Osminkina sought to figure out whether the silicon nanoparticles would indeed disintegrate and not remain in the system. To do this, she and her colleagues used two types of nanoparticles: one that was photoluminescent and one that was not. They used Raman spectroscopy, which involves shining a single color of light on a material and then deducing information about the material’s composition and properties by measuring how the light interacts with the material.
“This technique makes it possible not only to locate the nanoparticles in the cell (the signals from the silicon and cell components have different frequencies) but also to watch the process of their disintegration,” Osminkina said. “The latter was possible because, as already known, the Raman spectrum of silicon nanoparticles depends on their size—the smaller they are, the broader the spectrum becomes, shifting to lower frequencies.”
The researchers discovered that silicon nanoparticles sent to hunt down breast cancer cells localize on the cancer cell membranes within the first five to nine hours, then penetrate into the cells during the next day. Soon after penetrating a cell and delivering their cancer treatment, they start to biodegrade. This biodegrading is indicated by a decrease in signal amplitude, spectral broadening, and the appearance of the peak of the amorphous silicon phase. Finally, the experiments showed that by the 13th day after being introduced, the nanoparticles had dissolved completely and the signal had disappeared.
“Thus, for the first time we have shown that porous silicon nanoparticles could be completely harmless theranostics agents for many types of cancer,” said Osminkina. “They do not only easily penetrate into the diseased cell, but when filled with drug, can emit it during their dissolving.”
Dexter Johnson is a contributing editor at IEEE Spectrum, with a focus on nanotechnology.