In the early days of the coronavirus pandemic, it wasn’t just face masks and hand sanitizer that flew off drugstore shelves. Pulse oximeters were also in short supply, as news came out that a drop in blood oxygen could be a sign that a case of the coronavirus has taken a bad turn.
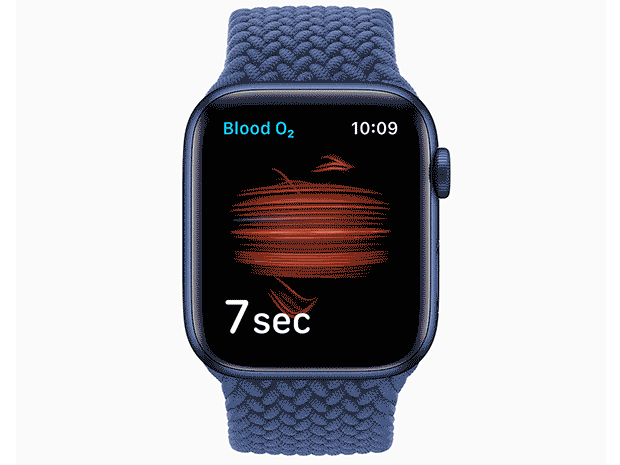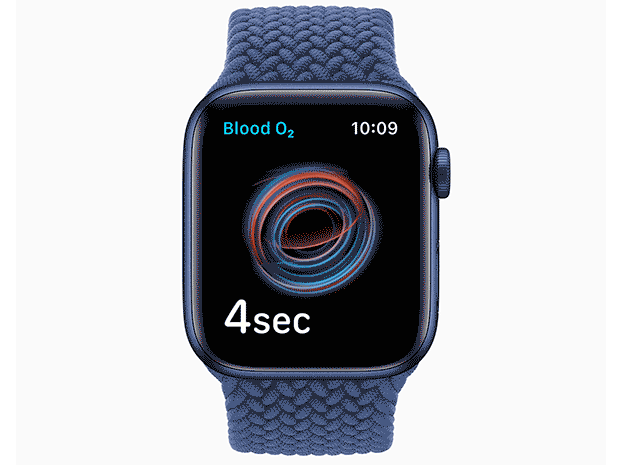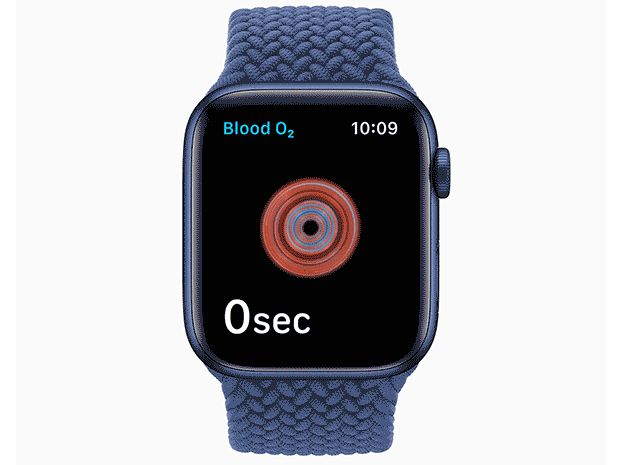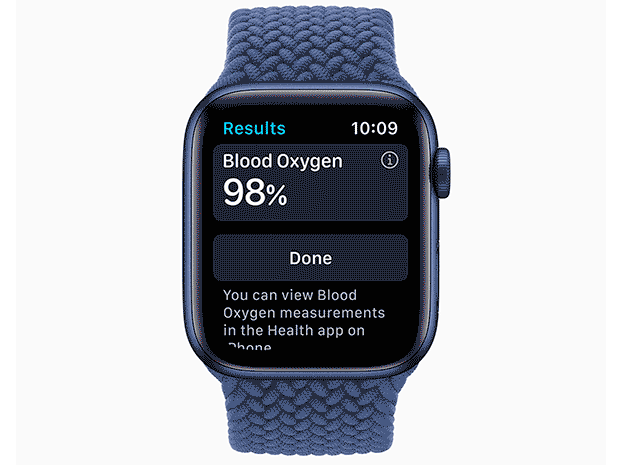
These inexpensive and noninvasive electronic devices use LED lights and photodiodes to determine the way red blood cells are absorbing light—oxygenated cells absorb more infrared light than red light, cells that aren’t carrying oxygen the opposite. With that information, algorithms can calculate a level of blood oxygenation; for most healthy people that’s in the high 90 percentile, in cases of COVID, the numbers dropped into the 80s. So it seemed like a good idea to have one on hand, if you could find one.
Now, six-plus months into the pandemic, it’s not surprising that consumer electronics manufacturers are touting the benefits of adding pulse oximeters to wearables. The sensors don’t cost much, they don’t use much battery power, and they could attract at least a few consumers looking to feel a little safer in this uncertain world.
Apple is the latest company to bring pulse oximetry to a wrist wearable (Fitbit and Garmin already had products out pre-pandemic, aimed at identifying sleep apnea). Announced last week, the Apple Watch Series 6 uses four groups of green, red, and infrared LEDs along with four photodiodes and what the company says is an advanced custom algorithm to determine blood oxygenation. (The red and infrared LEDs are involved in the oxygen measurement; the green LED can check pulse rate.) The sensors, mounted on the back of the watch and therefore touching the top of the wrist, can be used to take readings on demand during the day and automatically during sleep.
Apple is touting the gadget for “fitness and wellness.” Loosely translated, that means that this gadget does not have FDA approval to be marketed as a medical device. That comes as little surprise—FDA clearance takes time—but without that approval, it’s hard to know just how accurate it is.
Indeed, accuracy remains a question with many of the pulse oximeters on the consumer market. Says Steve Xu, a physician-engineer who is medical director for the Center for Bio-Integrated Electronics at Northwestern University, “It is relatively straightforward to make a pulse oximeter, even for an undergrad engineering design project, but it is really really hard to make a good one that is clinically dependable.”
Is Apple’s a good one? It’s hard to say just yet. Besides the issues of adjusting to different skin colors, coping with motion, and other design challenges faced by all pulse oximeters, putting the sensors on top of the wrist raises the difficulty level. The devices used in hospitals as well as the standalone gadgets sold in drugstores typically clip onto a fingertip or, sometimes, an earlobe.
“Those locations have an advantage over the back of the wrist,” Xu says, “because they have more capillaries, so provide a better signal to noise ratio.”
Wrist-worn blood oxygen sensors face another disadvantage: while fingertips are thin enough to allow light to shine through them, wrist oximeters must rely on reflected light, an inherently less precise approach.
“It’s not necessarily bad,” Xu says, “but all things being equal, isn’t going to be as accurate” as a pulse oximeter designed for a more favorable location.”
“I would never put a pulse oximeter on the wrist,” says William McMillan, co-founder, president, and chief scientific officer of Profusa, a company developing implantable biosensors. “The wrist is subject to a lot of motion, which is bad news for continuous measurement.” (Apple’s watches do have motion sensors which could help it identify quiet moments.)
Apple can show proof that its watch-based device can provide accurate oxygen saturation reading by taking it through FDA’s approval process, says Xu; the testing procedure for pulse oximeters is well-established.
Even without such vetting, Apple is launching several health studies using the Apple Watch oximeter—one looking at the management of heart failure, one at the management of asthma, and one considering blood oxygen level changes as early warning signs of COVID-19 and influenza. Both Xu and McMillan are cautiously optimistic about such efforts.
“Consumer product companies like Apple and Fitbit have a vastly bigger scale than most medical device companies,” Xu says. “Not many technology companies outside of the Apples and Fitbits and Samsungs can deploy a million devices in the world and manage the data that comes in. So we should do these studies and see how prognosticating they are, but we should realize there will be a lot of false positives. Maybe it will turn out to be a useful screening tool, but the verdict is still out.”
“Because [the device] isn’t likely terribly accurate, and it won’t involve a control group, rather, an uncontrolled situation, they will need a huge sample size to detect any phenomena,” says McMillan. “But they end up having millions of data points in which to look for insights, and that would be OK.”
This all begs the question—does the average smart watch user actually need to wear a pulse oximeter 24/7?
While such a wearable may tell us more about the fluctuations of blood oxygen saturation in the broad population, Xu says, “The vast number of healthy people are at 97 to 99 percent. If we all wear these and freak out whenever it drops to 92, the potential to worry people who are well is much higher than the clinical implications, which at this point are unknown.”+
Tekla S. Perry is a senior editor at IEEE Spectrum. Based in Palo Alto, Calif., she's been covering the people, companies, and technology that make Silicon Valley a special place for more than 40 years. An IEEE member, she holds a bachelor's degree in journalism from Michigan State University.