Using Lasers to Find Land Mines and IEDs
A laser could ionize a distant puff of air and thus safely detect the fumes from buried explosives

A mine-clearer's work still depends on metal detectors and sniffer dogs, with all the dangers that this entails. Technology may provide a better way to do the job.
Today we rely on dogs to sniff out hidden explosives. The problem is, you can't debrief a dog, so you can't identify the kind of explosive or even be sure that the animal is smelling explosives rather than packaging material. And who wants to risk the lives of dogs and their handlers? If you had an instrument that could safely identify any explosive at a distance—with the doglike power to detect molecules at concentrations of just one part in billions—you could get around these difficulties.
The problem of land mines is certainly not new, nor is even the problem of hidden homemade bombs, called improvised explosive devices (IEDs), although the latter came to prominence during the wars in Iraq and Afghanistan. Now these ghastly devices are proliferating around the world: The number of such bombings has increased from close to zero a decade ago to more than 4 000 per year in Afghanistan alone. It's a concern that will be with us for a long time, and as such it deserves serious efforts to address. Nor is the problem merely one of war and sabotage. Any device capable of sniffing explosives at a distance could also monitor all sorts of peacetime poisons and pollutants—carbon monoxide, mercury vapor, the oxides of nitrogen and of sulfur, and of course carbon dioxide and methane, the principal greenhouse gases.
We propose to find and identify such materials at a distance by using a laser to sample the spectroscopic fingerprints of trace gases in a distant volume of air. We use two complementary techniques to probe that volume: one involving a backward-propagating laser generated in the air sample itself, and the other a radar echo off ions and electrons from trace gas molecules that have been selectively ionized by a laser. At Princeton University we are examining both approaches because either one, taken alone, may sometimes be inconclusive and because at this early stage in development it's important to have more than one option. We've already achieved promising results in our research, which has been funded by the United States' Office of Naval Research.
What we want is a way to analyze the air remotely, without a physical sample, at a reasonable range—say, 30 meters—with high sensitivity and a low rate of false alarms. For this "standoff" capability we also need to put the transmitter and the detector together, with the detection signal returning to the spot where the initial burst of energy was emitted in the first place, as with a typical radar or sonar system.
There are many different ways a laser can sample the air above a suspected bomb. It can induce fluorescence or Raman scattering (which, like fluorescence, produces a signal with a distinct spectroscopic signature in the visible, infrared, or ultraviolet regions for each kind of molecule in the air). Or, if it's powerful enough, the laser can turn the air into a bright spark, so that its molecular constituents break apart and each element emits its characteristic spectrographic signature.
All these techniques produce light with telltale spectral features, but they don't work well at a distance. That's because the light the air gives off is incoherent, so it goes in all directions, and the intensity drops off rapidly with range. Also, the light is in the visible and infrared portion of the spectrum, so during the day, background sunlight tends to wash out the signal. Finally, there isn't much light to begin with—the molecules wafting from any explosives are so diluted by the surrounding air that only a few photons from them can be detected. Another approach, differential absorption light detection and ranging (known as DIAL), uses the backscattering from air, from particles in the air or from distant surfaces, to detect the absorption of a laser tuned to the proper wavelength and propagating through a cloud of the trace species. However, to make DIAL sensitive to within parts per billion requires paths of hundreds of meters through the cloud of trace molecules and entire minutes to process the data.
We propose instead to rely on coherent processes, in which the molecules act together as a marching band rather than as a milling mob. These processes include coherent anti-Stokes Raman scattering and coherent Stokes Raman scattering, which use laser beams to produce new laser beams that can indicate the presence of trace constituents. Both of these methods, however, have a serious drawback: The beams of laser light they create point away from the source lasers. To receive such a signal, you'd have to put the transmitter at one side of the target area and the receiver at the other—a most impractical setup in a battle zone. In addition, these techniques suffer from background interference from other coherent processes going on in the air, which tend to mask the signal coming from the material of interest, thus limiting the sensitivity you can obtain to a concentration of a part per million or so. That sounds pretty good, but in reality you need to be able to detect parts per billion, dog-nose style.
There is, in fact, a way to produce a laser beam that shines back at you, one bearing information you can use to identify the materials present at its source. By focusing a pulsed ultraviolet laser onto a targeted spot in the air, we have been able to create a region where the laser intensity is high enough to break up oxygen, freeing each molecule's two oxygen atoms. The beam is transmitted through a lens that focuses it on a spot 30 or so meters away; there it converges and then diverges, giving this region an hourglass shape. Because the laser pulse is very brief—on the order of nanoseconds—the intensity is high enough to break molecules of oxygen into their constituent atoms over a few millimeters in the thin midsection of the hourglass. This happens so rapidly during the pulse that the same pulse subsequently excites many of the oxygen atoms into a high-energy state, creating what's called a population inversion. This unstable condition then leads to the familiar laser chain reaction in which an excited atom drops to a lower energy state, emitting a photon of a particular wavelength, and that photon in turn stimulates another excited atom to emit a photon of the same wavelength and phase. These two photons go on to stimulate photon emission from two more atoms, and then there are four photons. The cascade amplifies the beam exponentially.
You might imagine that the laser light created by this process spreads out evenly in all directions—but no. Because the number of photons grows exponentially with distance, and because the lasing region is a few millimeters long and only a fraction of a millimeter across, the amplification is thousands of times as great along the length than along the breadth. This creates two beams, one that follows the path of the excitation laser and another that projects back in the opposite direction.
The beam that returns is, of course, the interesting one. There is no mistaking it for light that may have scattered straight back from the original beam, which at an ultraviolet wavelength of 226 nanometers is impossible to see. The return beam is also invisible, but it's at a wavelength of 845 nm, slightly too far into the infrared region to be visible. Still, that beam is remarkably bright, with a peak energy that's a million times as great as what you'd find in the scattered light.
Although this midair laser lacks mirrors—which would increase the efficiency of the lasing and channel all the output in one direction—its light nevertheless has all the characteristics of a true laser. First, the energy is concentrated into narrow, well-directed beams. Second, the pulse of light given off lasts less than a thousandth as long as the incoherent fluorescence that would have occurred were the oxygen atoms not lasing, so almost no atoms are left to fluoresce and almost all the energy goes into lasing. The atoms pour about 500 times as much energy into the return beam as into the fluorescence. Finally, the laser light is very monochromatic.
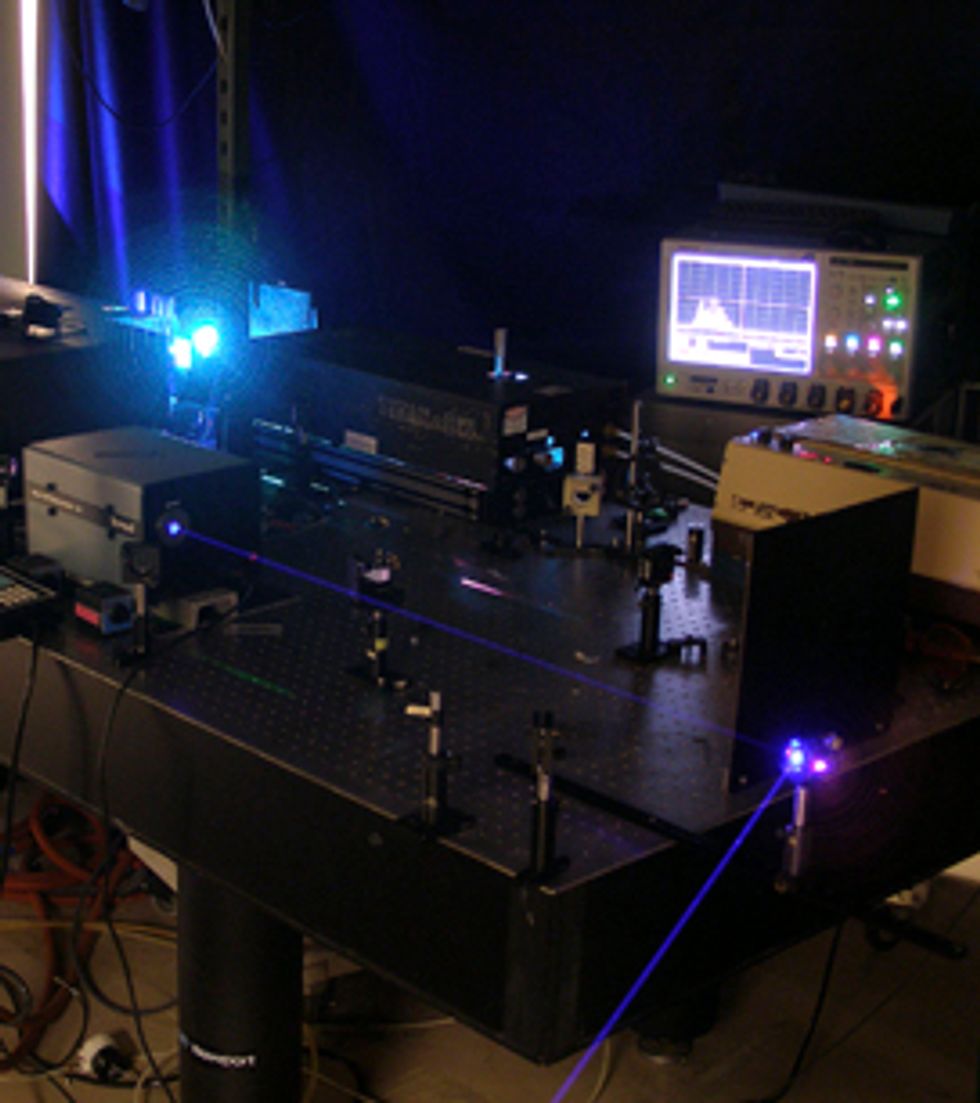
We get our ultraviolet excitation pulse by combining a pulse from a frequency-tunable laser with another pulse from a second laser and sending them together through a crystal with nonlinear optical properties. The crystal produces the required 226‑nm light. The return pulse from the air laser carries very little energy—a few hundred nanojoules, corresponding to a photon conversion efficiency of 0.1 percent or so. We can elicit a significantly more energetic return pulse by using another laser beam to create a spark in the air just before the ultraviolet pulse arrives. That way we prime the process with additional free oxygen atoms.
The brightness and spectral purity of the return beam make it easy to distinguish from scattered sunlight using nothing more than a simple filter. Also, because the beam arrives within a window of just a few nanoseconds, we can suppress noise even further by recording only the signal when the return beam arrives. This is too fast for regular camera shutters, but it's well within the capability of electronic shutters.
The idea of using one laser to create another dates back to the 1960s, when people began using dyes as lasing materials, exciting them with other kinds of lasers. The point was to make laser light of different wavelengths, and any loss in efficiency incurred by this two-step process was a small price to pay for that ability. Even mirrorless lasers are also well known—examples include the X-ray laser, the nitrogen laser, and naturally occurring interstellar lasers.
It occurred to us that we could use the two-step lasing process to identify trace molecules in the air. The trick is to exploit the way the returning air-laser beam behaves in response to changes in the outgoing pulse. The interaction is highly nonlinear: Even a small variation in the outgoing laser produces a big change in the return laser beam. If the ultraviolet excitation pulse is not focused tightly, the air laser does not produce as much light—and we can affect the focus using another laser.
We send this other laser beam out on top of the first one, and we tune the second laser so that its light will be absorbed by a specific trace molecule and no other. If the trace molecule is out there, waiting to be found, it will take in the energy and thus heat the air. Just a very little heating along the beam path makes the air behave as a lens, changing the focus of the ultraviolet laser. That change in focus then indicates that the molecule of interest is present. We establish that the focus has indeed changed by comparing the brightness of the backward laser beam altered by this telltale heating of the air with that of another laser beam that we send out simultaneously and focus on a nearby point.
Right now we're examining the processes involved in the formation of the backward laser beam. We've observed that the shape of that beam changes with the wavelength of the outgoing ultraviolet laser, but we still don't understand why. Also, the air-laser beam has a "spiky" nature, and we want to figure out where those spikes come from. Understanding these processes will allow us to minimize the pulse-to-pulse fluctuations of the backward propagating beam and improve the detection sensitivity of the air laser.
Because the detection of trace molecules is both difficult and important, it's a good idea to give bomb detectors in the field another method to double-check it. This backup method should be complementary, using a different physical phenomenon. We have opted for radar. Our technique is based on a phenomenon called resonance enhanced multi-photon ionization (REMPI), a very sensitive method used to detect trace molecules both in research laboratories and for such things as environmental monitoring and the analysis of coffee roasting.
In the standard REMPI process, a laser tuned to excite a particular energy transition in the target molecule is focused on one designated spot. If molecules of that kind are present, some will absorb a photon (or two, in the case of a two-photon transition), be excited to the higher energy level, and then absorb another one or two photons. When that happens, an electron is knocked out of the molecule, leaving it a positively charged ion. You can detect the presence of such ions either by seeing whether a current will pass through the gas or by running the gas through a mass spectrometer, which accelerates ions using an electric field and then bends their trajectories with a magnetic field, revealing their charge-to-mass ratios.
Our version, which we call radar REMPI, dispenses with the electrodes and mass spectrographs. Instead, we detect the charged particles using radar. The outgoing radio waves reflect off the electrically conductive region of ionization, just as if it were a metal particle, and return to the detector. We can achieve the same high sensitivity and spectral selectivity as with classical REMPI but at a distance. This approach serves as a complement to the air-laser method because it reveals the same target molecules in a completely different way. We have demonstrated its effectiveness by using the ultraviolet laser we employed to produce an air laser but tuned to a slightly different wavelength—one that excites nitric oxide through the absorption of a single photon. Once the nitric oxide is in that excited state, a second photon of the same wavelength can ionize the molecule. We used a very-low-power radar system—just 10 milliwatts—operating at 100 gigahertz and placed a few centimeters away from our sample, a blend of nitric oxide and air contained in a small glass vial, which kept the mixture controlled. The laser was focused on a very small volume within the vial, about 300 micrometers long and about 10 µm in diameter. Only the nitric oxide molecules in this tiny region were ionized.
The intensity of the return radar signal scaled with the concentration of the target molecule, all the way down to parts per million and below. We can even measure to better than the ambient 50 parts per billion concentration in room air. We began by placing the radar set just a few centimeters from the vial. Next we tried it a meter away—and then 10 meters away—and found that this system still worked very well. We expect that it'll function fine over some tens of meters—far enough away from a typical bomb to be safe. For those experiments we will use a higher power microwave, pulsed to coincide with the laser pulse.
Of course, the presence of nitric oxide doesn't prove there are explosives nearby. Nitric oxide is present in low concentrations even in clean air. Still, it does suggest the presence of nitrates, a common ingredient in explosives, and it is also the product of the laser-induced fragmentation of many other nitrogen-rich molecules found in explosives. We've already done some preliminary work that shows that we can distinguish such freshly made nitric oxide fragments from atmospheric nitric oxide molecules by their characteristic vibrations. Radar REMPI can also detect trace quantities of many other molecules of interest. This approach is good for standoff detection because of the high sensitivity of the radar process and its immunity to sunlight interference. And because the molecular selection (which is done with the laser) and the detection (which is done with the radar) are separate, you can transmit a very powerful radar signal without affecting the laser selectivity.
We are confident that the air laser and radar REMPI can each produce strong signals from trace elements that we can distinguish from background noise. Applying the techniques to the detection of IEDs will require much more work, because they have extremely low vapor pressures and are normally found outdoors, where wind and rain may obscure their presence. The system will need to operate at high repetition rates and with fast processing to scan large areas effectively. Also, because IEDs are frequently made from fertilizer, it will be difficult to distinguish them from organic waste.
Problems in the civilian world should, however, be much easier to tackle. Our two techniques could, for instance, be used to monitor air pollution, greenhouse gases, and gaseous leaks from chemical plants, and even for detecting the presence of natural gas. For these applications, all you have to do is detect gases whose concentration is in the parts per million, or even higher. And you often have to do the job in places inhospitable to humans—and dogs.
This article originally appeared in print as "Bringing Bombs to Light."
About the Authors
Richard B. Miles, Arthur Dogariu, and James B. Michael research optics at Princeton University. Miles is a professor of mechanical and aerospace engineering, Dogariu is a research scientist, and Michael is a graduate student. They measure subsonic and supersonic air motion with lasers and microwaves, accelerating air with surface electric discharges, magnetic fields, and electron beams, and speeding up flames with microwaves. Their work led them to use lasers for standoff detection. In short, "we work on air," Miles says.