The Incredible Pull of Nanocomposite Magnets
Nanotechnology could make rare earth magnets even stronger

In ancient times, they were considered magical objects with supernatural powers. These days, we just stick them on our refrigerators. Yet those little magnets deserve our admiration more than ever.
Take laptop computers, with their slim hard drives. It became possible to manufacture the motors for those drives only after the development of especially powerful permanent magnets in the early 1980s. Such muscular magnets are now found in many other places as well—various household appliances, cellphones, and the small electric motors that operate accessories in our cars, to name a few. They are also critical in the brawny electric motors that propel hybrid vehicles and in the generators attached to many wind turbines. So they can help both to reduce energy consumption and produce green electric power.
Because the magnets themselves are hidden away, many of us tend to take them for granted. We shouldn't, especially not now. The manufacture of most high-performance magnets requires neodymium, a rare earth element that's in short supply. Almost all of the world's production comes from China, which has increasingly restricted exports to ensure that it has enough to satisfy its own needs. So the price of neodymium has been skyrocketing. If the trend continues, pretty soon we'll have a real crisis on our hands.
Mining companies are scrambling to develop other sources of neodymium ore, a process that can take a decade or more. But what if you could make magnets even more powerful than today's best using less neodymium, or maybe even using an element that's a lot more plentiful? Not only would that ease concerns about shortages, it would result in smaller and more efficient motors and generators.
In the past, when researchers went looking for ways to make better permanent magnets, they were pretty much restricted to combining various mostly metallic elements into a single magnetic alloy. Now some of us are pushing an entirely different approach: We hope to construct ultrastrong magnets using two different alloys combined at the nanometer scale. That's not an easy thing to do, but if researchers can overcome the remaining technical obstacles, such "nanocomposite magnets" could allay worries over the dwindling supply of neodymium. And such magnets could make motors, generators, loudspeakers, and the like more compact and efficient, which is a worthwhile goal in itself.
How do you go about making a permanent magnet? Magnetism starts with individual atoms, so let's begin there. Only some kinds of atoms are magnetic, having what physicists call a magnetic moment, meaning that the atom acts like a tiny bar magnet. You'll definitely want to enlist atoms that are strongly magnetic in your creation. Iron, nickel, and cobalt all qualify. The rare earth elements neodymium and samarium are even better. The way those atoms are arranged is also important: You'll need a material in which these atomic bar magnets naturally tend to line up. This happens in many crystalline substances because of something physicists call exchange forces, a quantum-mechanical effect that operates over just a few nanometers, coaxing the magnetic moments of nearby atoms to align.
In many materials, that alignment is easily disturbed, destroying the magnetization. To avoid that, it's best to pick a material whose crystal lattice has just one direction that the magnetization can easily align with—think of the grain in a piece of lumber. Physicists call this, naturally enough, the easy direction of magnetization. The atomic bar magnets prefer that orientation to any other, which makes for a stable, or as physicists sometimes call it, a "hard" magnet.
But there's a complication. Most crystalline materials actually consist of many tiny crystallites packed together, so you'll likely be dealing with a bunch of little crystals, each with its own easy direction of magnetization. To get all their magnetic moments working in unison, you have to take pains to orient the easy directions of all the crystallites in parallel. Even if you're successful, the thing you create probably won't be uniformly magnetized. Instead, it will spontaneously divide itself into what are known as magnetic domains. The magnetic moments inside each domain will lie along the easy direction, but left to themselves, adjacent domains will point in opposite directions, canceling each other out.
This problem can be fixed by applying a strong magnetic field with a powerful electromagnet. As you turn up the current and apply a stronger and stronger magnetic field, the domains that are better aligned with that field will grow and eventually consume the other, less fortunate kind of domains. Once all the domains become fully aligned, the material reaches what physicists call the saturation magnetization. A good permanent magnet will keep most of that magnetization after the applied field is removed.
To find out how magnetically hard something is, physicists measure the strength of the coercive field, the reverse-directed magnetic field that's required to erase the material's magnetization completely. The greater the coercive field, the better, because magnets always work in a hostile magnetic environment, one that tends to demagnetize them. The rub here is that materials with strong coercive fields don't usually have much magnetic oomph—which is to say that at best, they exhibit modest saturation magnetizations.
Measuring the saturation magnetization and coercive field of a magnet tells you a lot about it. But what you'll really want to know after you've cobbled together a new magnet is a quantity called the maximum energy product. You can think of it as a measure of energy stored in 1 cubic centimeter of the magnetic material, although it's not stored in the sense that energy is stored in a battery or a capacitor—you can't just drain it off at will. And you wouldn't want to: The magnetic energy in 1 cubic centimeter of even a state-of-the-art magnet is surprisingly small—it could power a typical Christmas-tree bulb for only a fraction of a second. Yet the unique way this energy is retained within the material makes it truly indispensable, because it allows the thing to be permanently magnetic.
The maximum energy product of a magnet is often measured in units of millions of gauss oersteds, or MGOe. Its value determines the volume that a magnet must have to generate a magnetic field of a certain strength. The higher the maximum energy product, the smaller the magnet needed for a given purpose, whether it's spinning a motor, levitating a magnetic bearing, or sticking a calendar on the door of your fridge.
Physicists and materials scientists have invested enormous effort trying to find mixtures of elements that produce both high saturation magnetizations and strong coercive fields. The best results have come from using various combinations of samarium and cobalt or of neodymium, iron, and boron. Samarium-cobalt magnets have better temperature stability, but they are expensive, and their maximum energy products are only some 20 to 30 MGOe. Neodymium-containing magnets often have energy products of 40 to 50 MGOe and are less costly, but they can't take heating nearly as well.
Neodymium magnets have improved steadily over the three decades since they were invented, but they're now reaching their limits. Materials of this type have a theoretical maximum energy product of 64 MGOe, and scientists from Neomax Materials Co., in Japan, have already produced a neodymium magnet with 92 percent of this value. There's just not that much room for improvement.
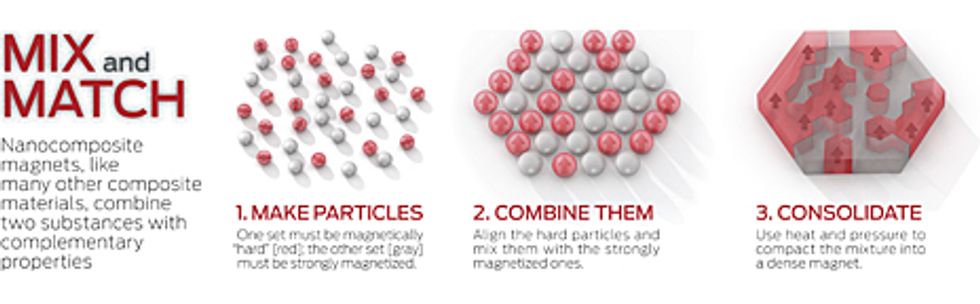
Some yet-undiscovered combination of elements may well beat out neodymium-iron-boron. But searching for a new wonder substance isn't the only path to a great magnet. Around 1990, a group of Soviet researchers led by Nikolay Manakov, a physicist currently at Orenburg State University in Russia, and independently, Eckart Kneller and Reinhard Hawig from Ruhr University in Germany proposed an altogether different approach. Their idea was to juxtapose two different magnetic materials, one having a high coercive field, the other a high saturation magnetization. If this was done on a fine enough scale, they thought, exchange forces from the former would magnetically stabilize the latter. As with more familiar composite materials, two different components mixed together can serve much better than either one can alone.
For a composite magnet to work, the high-saturation material can't be any more than 10 or 15 nanometers thick. Otherwise, exchange forces won't reach far enough into its interior. Although no such restriction exists for the stabilizing component, you won't get much of it adjacent to the high-saturation stuff unless it's finely broken down, too.
Such nanocomposite magnets promise higher saturation magnetizations—and in turn, higher maximum energy products—than today's best neodymium magnets. As far back as 1994, researchers calculated that using an iron-cobalt mixture for the high saturation magnetization it provides and stabilizing it with a samarium-iron-nitrogen alloy could produce magnets with maximum energy products as large as 137 MGOe—more than twice the current record. In addition, such magnets would require much smaller amounts of rare earth elements and would better resist corrosion, a notable problem with today's high-performance magnets.
You might guess that mixing two different metallic alloys on the nanoscale would require ultramodern fabrication techniques. In fact, materials scientists have long known how to get such combinations to form without having to create them from nanometer-size building blocks, and researchers have explored those techniques—including ultrafast solidification, mechanical alloying, and sputtering—to fabricate nanocomposite magnets. But so far, the resultant maximum energy products haven't approached the whopping values theorists predict are possible.
One problem is that it's almost impossible to make the bits of the high-saturation material small enough and to position them so that they're always surrounded with high-stability material. The other stumbling block is the poor alignment—or even the complete lack of alignment—between the easy directions of magnetization in the high-stability material. And when the theorists calculated those stupendously high values for the maximum energy product, they assumed perfect alignment.
Given these challenges, it's no surprise that past attempts have failed to create a good nanocomposite magnet. That's why in recent years researchers have begun thinking seriously about producing such a magnet from the bottom up—that is, by first synthesizing nanometer-size magnetic particles and then assembling them.
Chemical techniques could work for that. In 2000, for example, a group of scientists at IBM under the direction of Shouheng Sun chemically synthesized high-saturation-magnetization nanoparticles of an iron-platinum alloy, a rather exotic (and extremely expensive) permanent-magnet material. That encouraged other chemists to try to do the same for samarium-cobalt and neodymium-iron-boron alloys. So far, those researchers have had no luck. Recently, Sun, who is now at Brown University, and J. Ping Liu, from the University of Texas at Arlington, have managed to synthesize samarium-cobalt nanoparticles, but those particles were stuck together in clumps, making their use in nanocomposite magnets problematic.
We are also trying to build composite magnets from the bottom up. But we make nanosize magnetic particles using a variation on a standard metallurgical technique: ball milling. When a powdered substance is stirred, tumbled, or shaken in a vial filled with steel balls, the particles usually break up but sometimes stick together. The trick is to do the milling in a liquid that resembles gasoline, to which a small amount of a fish-oil-like surfactant is added. That greatly reduces the tendency of the particles to stick together, because the surfactant molecules surround the particles, keeping them apart.
Such surfactant-assisted ball milling can produce magnetic particles that are only a few nanometers across. We have also managed to obtain samarium-cobalt flakes that are a few tens of nanometers thick, which should work well as the high-stability component in a composite nanomagnet. We can easily produce large quantities of these magnetic flakes, but we continue working to control their sizes better and to figure out ways to protect them from oxidation, which acts fiendishly fast with things this tiny.
Our ideas for how these particles could be combined with others of high saturation magnetization and how the resulting mixture could be consolidated into dense nanocomposite magnets are admittedly somewhat sketchy. Twice, in 2002 and in 2007, groups that included researchers from Georgia Tech, IBM, Iowa State University, Louisiana Tech University, the University of Texas, and Chiba Institute of Technology, in Japan, employed similar techniques to fabricate nanocomposite magnets. But they used two different iron-platinum alloys, which resist oxidation. Because those particles were assembled randomly and because the chosen high-saturation particles had a magnetization that wasn't all that high, the magnets they made didn't perform well.
One relatively simple way to assemble these two kinds of particles is by depositing the high-saturation ones as a thin coating on high-stability cores. The easy direction of magnetization of the cores would have to be aligned in an applied magnetic field before the mixture was compacted. The particle coatings would then merge into a network of thin layers. In an experiment carried out in cooperation with Electron Energy Corp. of Landisville, Penn., we tested this idea by coating relatively coarse copper particles with iron nanoparticles. After compacting them at high temperature, we observed just such a network of thin iron layers inside a piece of copper. Copper isn't magnetic, though, so the result wasn't a permanent magnet. But this experiment provided a convenient way to test our ideas and procedures.
The problem with this approach is that it works well only when the cores dwarf the particles in the coating. This means that only a small amount of the high-saturation material can be added. So any nanocomposite magnet made this way, assuming that all other difficulties are trumped, would not be much better than magnets made from whatever material is used for the cores.
We and other researchers clearly need to explore other approaches if we truly want to make real strides. One possibility is to use surfactants more cleverly so that one set of magnetic particles becomes negatively charged on their surfaces while the other set becomes positively charged. Mixing the two kinds of particles in a liquid in the presence of a magnetic field might then give the desired arrangement, with particles of one type alternating with particles of the other, with the easy directions of magnetization neatly lined up.
The problem with using a surfactant to assist in assembling nanoparticles is that the molecules of the coating would be very much out of place in the final magnet. At best, they would dilute its magnetization. At worst, they might even react with the rare earth element, destroying its stabilizing effect. So we would expect that the surfactant molecules would have to be removed after assembly, either by dissolving them chemically or by heating the mixture until these molecules decompose or evaporate.
Assuming that the desired nanoparticle assemblies could be arranged in this way, the results still wouldn't make for very good permanent magnets: They would neither be mechanically strong nor sufficiently dense. Somehow you'd have to compact them, a step that we don't think would be any easier than the preceding ones. If you use only pressure to do that, for example, the pressures would need to be impractically high, because hard alloys like samarium-cobalt are exceedingly difficult to pack together. You'd need to use explosives to achieve the required amount of squish, and that doesn't make for an orderly assembly line! A combination of pressure and heat would probably work better, but heating might coarsen the particles or initiate unwanted chemical reactions.
This list of obstacles gives you an idea why progress in the not-so-young quest for nanocomposite magnets has been advancing so slowly. But thanks to more than a few incremental successes, we not only understand what we want to achieve, we also clearly see a path to getting there. There is a feeling within the community of researchers working on this problem that any day now our efforts will be rewarded with an impressive next-generation supermagnet. And even if that great magnet of the future proves a long time coming, we are still bound to find a way to make it, one tricky step at a time. Get your refrigerator ready.
This article originally appeared in print as "Magnet Makeover."
About the Authors
George Hadjipanayis and Alexander Gabay have worked for a decade to make stronger magnets. Hadjipanayis chairs the physics and astronomy department at the University of Delaware, where Gabay is a research scientist.