Why Sweat Will Power Your Next Wearable
Biofuel cells can generate enough watts for fitness trackers and health monitors

Admit it—you love your smart watch and all the amazing things you can do with it. But the task of keeping it charged can be annoying. A longer battery life would be great—but with batteries, the amount of energy stored correlates with volume, and bigger batteries add bulk and weight. All wearables, including fitness trackers, audio-enhancing hearables that go in your ear, and augmented-reality contact lenses, have a similar power problem. And today’s batteries are too bulky and stiff for use in wearables that are woven into textiles or directly mounted on a user’s skin.
The power demands for these kinds of devices range from 1 milliwatt for a basic step counter to tens of milliwatts for more advanced smart watches. When using small, centimeter-size batteries, which have capacities on the order of 10 to 300 milliampere hours, this results in battery lifetimes of only a few days at most.
Some researchers are tackling the wearable power challenge by developing new types of stretchable batteries and supercapacitors. However, it’s hard to produce such batteries using screen printing, a process that dramatically lowers costs. Other developers are trying to bypass batteries altogether by using Near Field Communication chipsets for wireless power transmission. But NFC technology requires you to have an external power source, like a mobile phone, within a few centimeters of the wearable; once you move the phone away, the wearable stops working.
At the Center for Wearable Sensors at the University of California, San Diego, we think there’s a better way to power the next generation of wearables: scavenging energy—especially biofuels--from the wearers themselves. Devices powered this way could be so small that you’ll forget they are there. We call them “unaware-ables.”
And the first practical biofuel will be your own sweat.
Harvesting energy from your body or your surroundings isn’t a new idea. Earlier approaches involved tapping into motion, light, and heat. Since the 1770s, for example, self-winding watches have used the natural motion of the body to scavenge power. In the first such devices, a weight inside the watch would wind the mechanism. Later versions had magnetic weights that passed through a coil, which generated electricity to charge a battery. Modern self-winding watches use piezoelectric materials, crystalline substances that release an electric charge when bent or squeezed.
Scavenging light is done with fingernail-size photovoltaic cells, which have been used to power calculators for decades. Most are rigid, but researchers in our center are developing solar cells that are flexible and even stretchable, to better conform to the human body.
Body heat is a third energy-scavenging approach. In most climates and settings, the temperature of the human body is well above ambient temperature. Tiny thermoelectric generators exploit this differential. The body-heat scavenger used on the PowerWatch, which is a fairly basic smart watch, fits on the skin-facing side of the device.
Unfortunately, few of these tried-and-true energy-harvesting methods have been able to supply sufficient power for a small, flexible, useful wearable. Motion harvesters are fine for watches, but there’s not enough motion to harvest in the areas where the newest wearables are worn, such as on the chest or in the ear. Thermoelectric generators can scavenge energy effectively only when their design includes a large heat sink, typically made from aluminum, to collect the body’s heat and transfer it to the device. And thermoelectric generators and solar cells don’t work well if the wearable is worn under clothing.
That’s why we’re so excited about sweat power. Certain chemicals found in human sweat can be used as fuel in wearable-size fuel cells. These biofuel cells could offer high power densities in a more practical, wearable form than is possible with any of the existing energy-scavenging approaches. Our team has already developed prototype wearables that generate power from sweat.
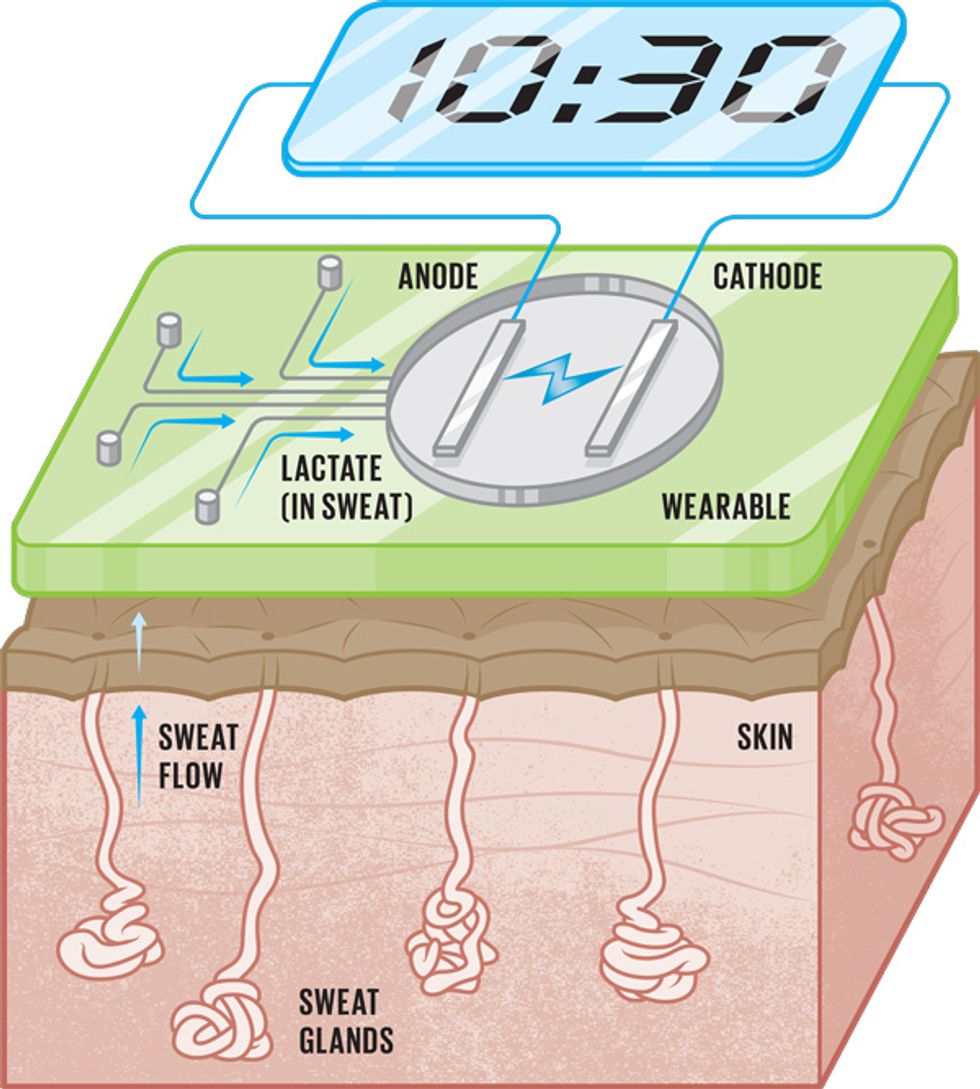
Here’s how a wearable turns sweat into energy: A fuel cell consists of two electrodes—an anode and a cathode—with an electrolyte between them. The fuel goes into the anode, where a catalyst separates its molecules into electrons and protons. The protons pass through a membrane to the cathode, while the electrons flow into a circuit. The Welsh scientist William Robert Grove first worked out the process in 1839; he used hydrogen as the fuel and oxygen as the catalyst to generate water and electrical current.
Hydrogen is impractical for a wearable fuel cell because it’s highly flammable. Sweat, on the other hand, is easily acquired and abundant, particularly when a person is exercising or playing sports. And, given that athletes embraced all sorts of wearables early and widely, they represent an attractive early market for sweat-powered devices.
Sweat isn’t just water. It contains trace amounts of a wide variety of minerals and other substances like glucose and lactate. These substances, called metabolites, are by-products of the chemical processes that constantly go on inside living beings, and they make attractive biofuels. We are particularly interested in lactate, because its concentration in sweat rises with exertion. In our sweat biofuel cells, we create a layer of enzymes that reacts with the lactate in sweat to split the electrons and protons and create an electrical current.
We’re not the first researchers to think of using body fluids as fuel. Some of the original pacemaker and cochlear implants proposed in the 1970s were intended to use glucose biofuel cells for power. Given the abundance of biofuels inside the body, using them for implantable devices was a logical choice. The main drawback was that the enzymes used to catalyze the fuel cell reaction would degrade, and the electrode would stop functioning within a few days. The only way to restore the fuel cell’s operation was to surgically remove the implant, which was obviously impractical.
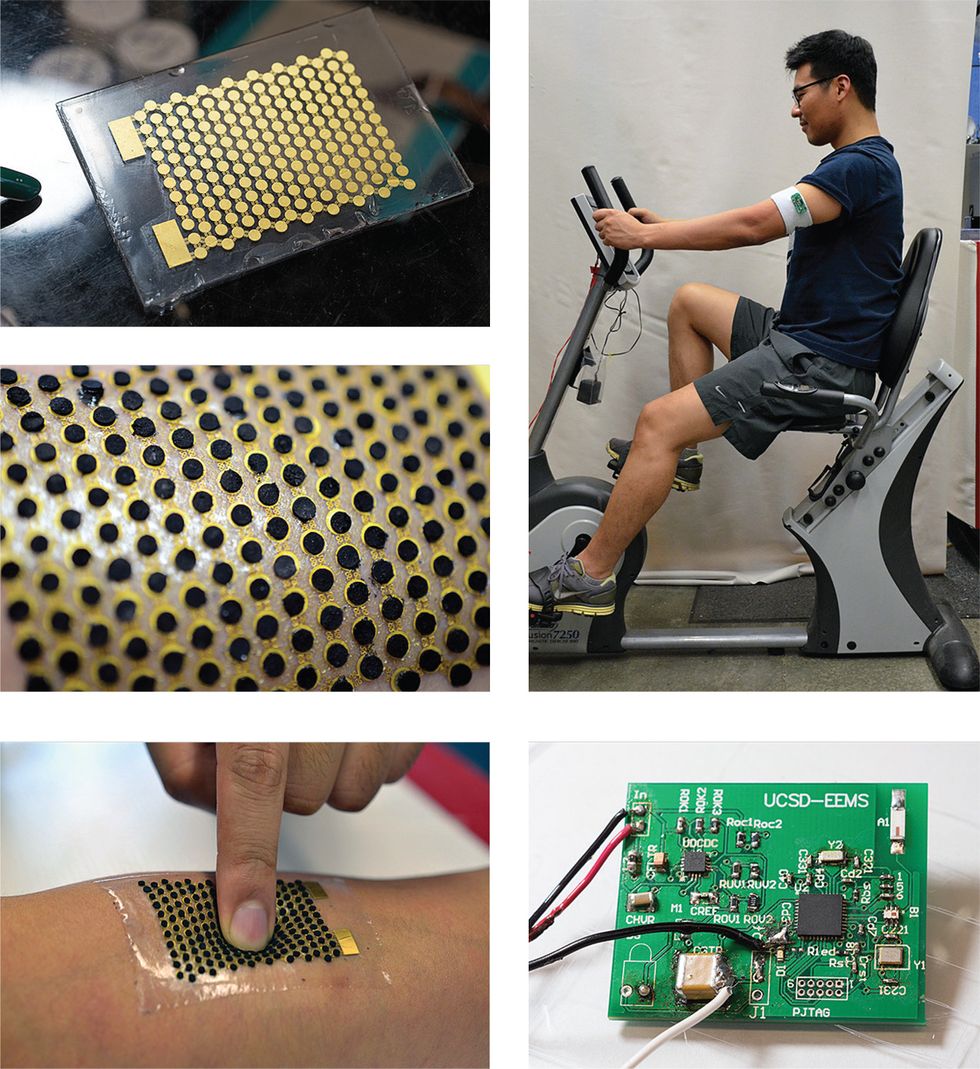
To avoid the enzyme-depletion issue, our group instead has focused on developing disposable wearables worn outside the body. We demonstrated our first biofuel cells in 2014. The lactate biofuel cells were screen-printed onto a fabric headband and a wrist-worn sweat guard.
Our test subjects each wore a headband and a sweat guard while riding an exercise bike. We’d hooked up each biofuel cell to a small DC-DC converter. The converter raised the voltage generated by the perspiring bike riders to the levels necessary to light up a microwatt-level LED and power a digital wristwatch. In the experiment, the biofuel cells generated up to 100 microwatts per square centimeter, enough to power both the LED and the watch. That’s a greater power density than would be possible with a thermoelectric generator paired with a small, wearable-size heat sink, and somewhat greater than the power density of a solar cell operating in normal indoor lighting. (We did not measure lactate concentrations during the experiment; these vary by subject. Our previous, in vitro experiments, which produced similar power levels, used lactate concentrations of 3 × 10–3 moles per liter (3 moles per cubic meter) to 15 × 10–3 mol/L (15 mol/m3).
To our knowledge, this was the first demonstration of a printable, wearable biofuel cell powering an everyday object. What’s more, because the biofuel cell was printed onto a flexible textile, the design was reasonably comfortable to wear, and it continued to function even after repeated bending. However, it didn’t generate quite enough power to operate a sophisticated activity tracker or a full smart watch. It also didn’t conform to the skin as comfortably as we would have liked.
Of course, truly useful wearables have more components than a digital watch does. Even the simplest activity tracker includes an accelerometer, memory, and a Bluetooth radio. Together, these items consume about a milliwatt or two, about 10 times as much as what we generated in our 2014 demonstration. Indeed, boosting the power density of wearable biofuel cells is one of our biggest challenges. If the cells can’t generate much more power than alternative technologies can, they won’t make it into real products.
In reviewing our fuel cell’s design, we realized that the surface of the anode was relatively flat. That meant the anode was exposed to only the molecules resting directly below it, which greatly limited the amount of biofuel the cell could catalyze. In 2017, in collaboration with our UC San Diego colleague Sheng Xu and his team, we came up with a 3D carbon-nanotube structure in the form of pellets that could be attached to the top of the anode and the cathode. These pellets increased the electrodes’ effective surface area without increasing the actual area of the device. Although using this 3D structure meant our cell was no longer fully printable, it allowed us to load each electrode with more of the catalyst, giving access to more fuel and enabling each cell to generate much more power.
Increasing the cell’s effective surface area and making additional improvements in the chemical composition of our catalyst enabled us to boost the power density by a factor of 10, to 1 milliwatt per square centimeter. That power density approaches that of a small solar cell in direct sunlight.
To test this design, we built a circuit board that included a Bluetooth radio, typically the most power-hungry component of a wearable device. Our prototype lactate fuel cell generated around a milliwatt, enough to successfully power the radio, a small microprocessor, and peripheral components, including a temperature sensor and voltage converter.
The 3D electrodes we used in this design weren’t flexible. Ultimately, we want a fuel cell that’s not just flexible but also stretchable. The human body is bumpy and curvy, so flexibility alone isn’t sufficient to ensure that the fuel cell will conform smoothly to a user’s skin—think of trying to wrap a sheet of paper around your wrist. Something stretchy like nylon or cling wrap would be much better.
To create flexibility and stretch, we again turned to carbon nanotubes, using an “island bridge” structure developed by Xu’s group. This biofuel cell is dotted with rows of “islands,” each one of which is a 3D electrode. Half of the islands make up the anode, and the other half form the cathode. The islands are connected by stretchy “bridges” made of thin, spring-shaped wires that can stretch and bend. However, the electrodes themselves don’t stretch, so over time, the mechanical mismatch can create a lot of strain and cause the fuel cell to fail. Our team is currently working to solve this problem.
There’s one more hurdle to bringing sweat power to wearables: In most situations, people don’t sweat constantly—or at least not heavily enough to generate much power. If you’re not sweating, your fuel cell will run dry and stop producing power. This may not be an issue in applications like exercise and athletics, but it’s a big deal in most other cases.
There are three ways to work around this limitation. We could use the scavengers only for applications where the availability of sweat is guaranteed. Or we could add an energy-storage element to the wearable. Or, finally, we could add a complementary, nonbiofuel energy scavenger to the wearable.
We don’t want to limit the applications to athletes in action, so we’ve been focusing on the two latter approaches. For wearables that need a constant supply of energy—for example, smart watches—an obvious solution is adding a battery or an ultracapacitor to act as an energy buffer. If the fuel cell has a high power density but the availability of power is intermittent, then the wearable device will charge its battery when power is available and discharge the battery when the biofuel cell stops producing power.
This energy buffer needs to have the same general physical properties as the rest of the wearable. It doesn’t make sense to have a biofuel cell that’s small, soft, and stretchable if the battery on top is large and rigid.
Last year, researchers in our center created a stretchable textile that uses sweat to feed its biofuel module and stores the energy in a flexible supercapacitor. We’ve also demonstrated stretchable and rechargeable zinc-silver-oxide battery prototypes, made using printed electronics. These stretchy batteries can be recharged hundreds of times and can generate relatively high currents, although their energy densities have a long way to go before they can match those of their rigid counterparts.
To increase the likelihood of successful energy harvesting and therefore decrease the demands on the energy buffer, we’re also looking at using multiple types of energy scavenging to power a single wearable. Users may not necessarily sweat all the time, but if their wearable combines a biofuel cell with a solar cell and a thermoelectric generator, then the chances of any one of these devices generating power at a particular time is going to be higher than with one of them acting alone.
Of course, integrating several scavenging technologies needs to happen without compromising the wearer’s comfort. As with the biofuel cell–energy buffer combination, we have to make these gadgets small, soft, and stretchy. Researchers have lately been considering how to redesign energy-scavenging devices for better integration into what we like to call an energy sandwich—that is, a collection of energy scavengers stacked in a single small, stretchable device. In 2018 we developed a small circuit that can simultaneously extract energy from multiple sources and send it to multiple wearables and a battery simultaneously.
So far, we’ve been talking about using biofuel cells to replace traditional batteries in wearables. UC San Diego researchers are also making progress on printable batteries that are smaller, cheaper, and more flexible than traditional batteries. These, along with supercapacitors, will eventually be useful for briefly storing the energy harvested by wearable biofuel cells.
And here’s the real magic of biofuel cells: They can act as sensors. That’s something no battery can do.
The sensing is possible because the output power of a biofuel cell is usually proportional to the underlying concentration of metabolites being used as fuel. We can exploit this phenomenon to build self-powered sensors—devices that perform sensing and energy harvesting at the same time. This idea was first reported in 2001 by a team led by researchers at the Hebrew University of Jerusalem.
Our team has developed self-powered biosensors to detect changes in the lactate or glucose concentration of sweat while using that same lactate or glucose to generate the power the sensor needs to operate. Lactate is a good marker of exertion, and knowing one’s level is useful for athletes and also for people fighting illness. Glucose is a marker for nutrition as well as for blood sugar levels. Custom low-power microelectronic circuits in the device—powered by the biofuel—read the sensor data, convert it to a digital format, and wirelessly transmit the reading to a smart watch or phone. We believe this is the first practical demonstration of a noninvasive, integrated, self-powered chemical biosensing system with a wireless readout.
Sweat power is a new technology, and many challenges remain. For one thing, not everybody sweats in the same amounts; we have to make sure our systems can adapt to varying conditions. We also need to do a better job of integrating these biofuel cells with other harvesters, energy sources, and electronics to create useful wearables.
And finally, the longevity of our biofuel cells needs improvement. Right now, most of our prototypes are designed for use in disposable devices, as easy to put on and take off as a Band-Aid. These biofuel cells might go into wearables meant to be worn for a day at most, for use by athletes trying to optimize training, people fighting illness who need to monitor temperature and hydration, or those just interested in tracking their general well-being. While we feel this approach is reasonable for many applications, we’d still like to try to extend the devices’ longevity a bit further.
If we do our job well, we will create a class of small, flexible, robust, rugged, washable wearables that can be used around the clock and never need to be recharged. Sweat isn’t the only biofuel—our bodies produce a variety of potential biofuels. Tears could power smart contact lenses, while saliva could power smart mouth guards. A baby’s urine could power a smart diaper, and fluid collected from the skin using microneedles could power a drug-delivery device. These and other self-powered biosensors would measure myriad biological parameters and report their data wirelessly to a user’s health app, yielding far better information than what’s now available from traditional wearables. That’s our vision of an “unaware-ables” future.
This article appears in the July 2020 print issue as “Powered By Sweat.”
About the Authors
Patrick Mercier is an associate professor of electrical and computer engineering and Joseph Wang is a professor of nanoengineering at the University of California, San Diego. Mercier and Wang are also co-director and director, respectively of the university’s Center for Wearable Sensors.