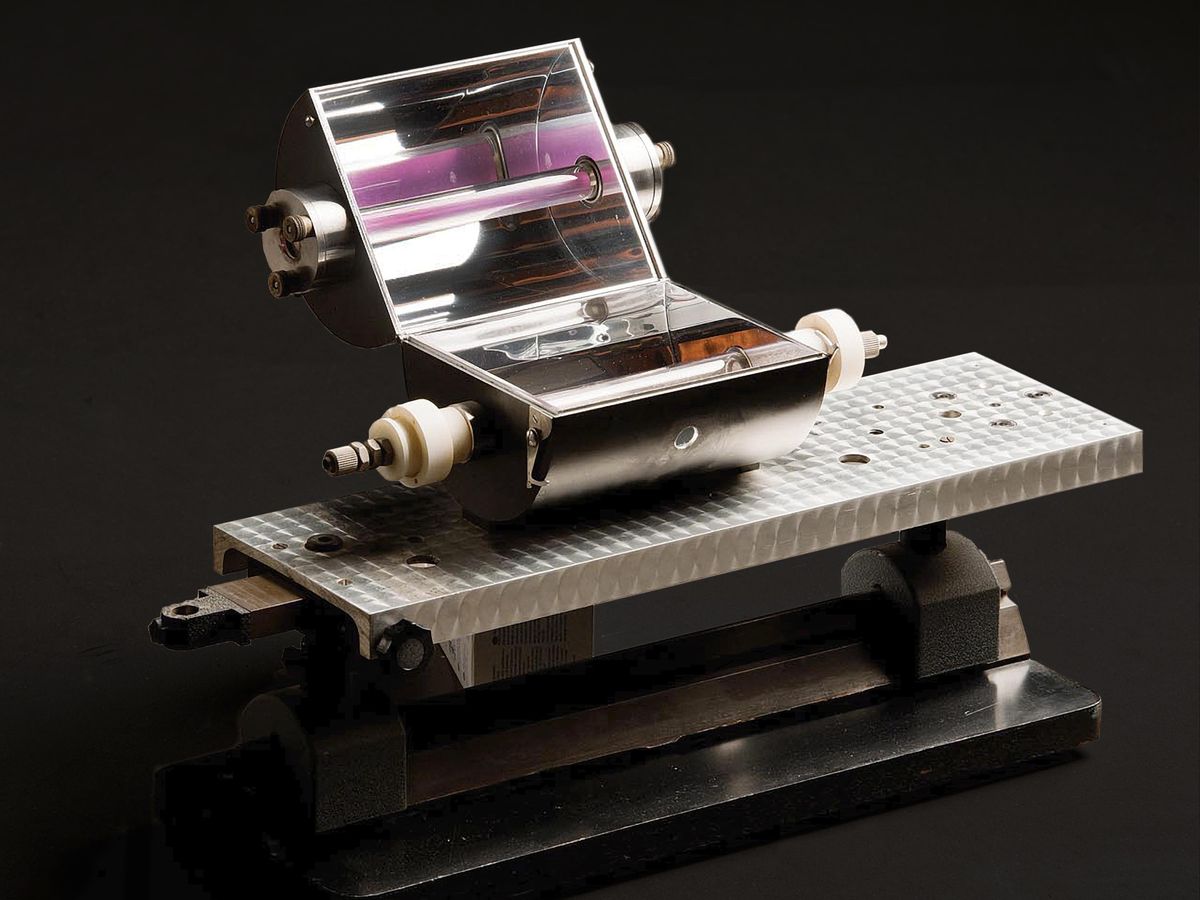
When the future Nobel-winning chemist George Porter arrived as a Ph.D. student in chemistry at the University of Cambridge in 1945, he found the equipment there “remarkably primitive,” as he told an interviewer in later life. “One made one’s own oscilloscopes.”
Porter’s adviser, Ronald G.W. Norrish, ran a lab within the Cavendish Laboratory that had its share of jerry-rigged equipment, and he assigned Porter a problem: Establish a technique for detecting the short-lived molecules known as free radicals. Porter already knew a fair amount about not just chemistry but also physics and electronics. He’d spent most of the war with the British navy working on applying pulse techniques to radar.
Porter began by investigating the methylene (CH2) radical, using the lab’s surplus army searchlight. It ran on 110 volts DC supplied by a large army diesel engine that sat on the back of a truck parked outside. One of Porter’s jobs was to hand start the engine on cold winter mornings.
Detecting free radicals was no mean feat, as they typically exist for milliseconds or less. At the time, chemists barely used the word millisecond. At a science conference in September 1947, the prominent chemist Harry Melville stated that specimens with lifetimes of less than a millisecond were far beyond direct physical measurement. Porter and Norrish were about to prove him wrong.
George Porter’s flash of insight at a lighting factory
On a trip to collect a mercury arc lamp for the searchlight, Porter saw flash lamps being made at a Siemens factory. The figurative lightbulb turned on. He realized that combining flash lamps with pulse techniques he’d learned as a wartime radar officer could be applied to his current problem. In the lab, they had been using a continuous light source without much success. Porter reasoned that they could use a more intense pulse of light to excite the sample and create the free radicals, and then use further flashes to record the decay. He began experimenting and soon developed the technique he dubbed flash photolysis. It reset the time scale for chemistry and revolutionized the field.
After completing his thesis, “The Study of Free Radicals Produced by Photochemical Means” in 1949, Porter stayed on at Cambridge, first as a demonstrator and then as the assistant director of research in the Department of Physical Chemistry. In 1954 he left Cambridge to become assistant director of the British Rayon Research Association, but he soon realized he was better suited to academia than industry. The following year he became the first professor of physical chemistry at the University of Sheffield.

Porter’s research into ever-faster chemical reactions easily progressed from milliseconds to microseconds, but then things stalled. He needed a faster light source. When Theodore Maiman demonstrated a laser at Hughes Research Laboratories, in California, in 1960, Porter immediately realized it was the light source he’d been waiting for. Britain was behind the United States when it came to laser research, and it took time to acquire such an expensive piece of equipment.
Once Porter had his ruby laser [pictured at top and now on exhibit at the Royal Institution], he lost no time in pushing his research into the nanosecond and picosecond region. In 1967, Porter and Norrish along with the German chemist Manfred Eigen were awarded the Nobel Prize in Chemistry, for their studies of “extremely fast chemical reactions, effected by disturbing the equilibrium by means of very short pulses of energy.”

Later in his career, Porter moved to Imperial College London, where he had access to more advanced lasers and was eventually able to capture events in the femtosecond range—12 orders of magnitude faster than during his doctoral studies at Cambridge. Flash photolysis is still used to study semiconductors, nanoparticles, and photosynthesis, among other things.
Porter viewed his contributions to photochemistry as essential to the future of the planet. As he told an interviewer in 1975, “Our future, both from a food and an energy point of view, may well have to depend largely on photochemistry applied to solar energy. The only alternative is nuclear energy, which certainly has its problems, and it would be wise to have something else up our sleeves.”
How George Porter became a popular popularizer of science
Porter wasn’t just interested in pushing the boundaries of science. In 1960, he began giving the occasional public lecture at the Royal Institution, in London, filling in for speakers at the last minute. This eventually led to his appointment as a professor of chemistry at the RI, a part-time post with a tenure of three years that consisted of giving a public lecture and a few school lectures each year. This arrangement allowed Porter to develop a relationship with the institution while keeping his position at Sheffield.
In 1966 Porter became director of the RI’s Davy-Faraday Research Laboratory, otherwise known as the DFRL, and permanently moved his research from Sheffield to the institution. He was also named the Fullerian Professor of Chemistry and the overall director of the Royal Institution. The Science Research Council provided Porter with funds to support a research group of approximately 20 people, and he spent the next two decades at the RI as an active researcher, as well as a popularizer of science. He excelled in both roles.
The late 1950s and early 1960s saw intense public and academic discussions about science education and science literacy. In a series of provocative newspaper articles and a 1959 lecture at Cambridge entitled “The Two Cultures,” the novelist and physical chemist C.P. Snow argued that the British educational system favored the humanities over science and engineering. He stoked outrage by asking educated individuals if they could describe the second law of thermodynamics, which he believed was the equivalent of being able to quote Shakespeare. Academics and public intellectuals from both the humanities and the sciences took up the debate, some earnestly, others with derision.
Porter deftly threaded the needle of this debate by slightly reframing the analogy. Instead of Shakespeare, Porter used Beethoven. He argued that anyone could appreciate the music, but only a musician who had studied intensively could fully interpret it. Similarly, to understand the second law of thermodynamics, it might take years of specialized study to derive intellectual satisfaction from it, but people needed only a few building blocks to get the gist of the physics. Porter chose to bridge the cultural divide with a commitment to explain science to nonspecialists.
As if to prove his point about thermodynamics, Porter wrote and presented a 10-part TV series called “The Laws of Disorder” for the BBC. (He had already established himself as an excellent science communicator in an episode of the BBC’s “Eye on Research.”) In this episode, he tackled the second law head on:
The Second Law of Thermodynamics - Entropywww.youtube.com
According to historian Rupert Cole in his 2015 article “The Importance of Picking Porter,” managers at the Royal Institution recognized Porter’s ability to engage with the “Two Cultures” discourse and to explain basic science in an accessible manner when they selected him as director.
One of the RI’s great traditions was to explain science to the public. Porter regarded the institution’s theater as the London repertory theater of science. Under his leadership, the institution welcomed school children and laypeople to learn about the latest developments in science. He also insisted that speakers use demonstrations to help explain their work. At the end of his tenure there, he recorded a number of famous experiments. He also worked with BBC television to broadcast the Christmas Lectures. In this clip, Porter demonstrates how to make nylon:
How Nylon Was Discovered - Christmas Lectures with George Porterwww.youtube.com
The Christmas Lectures, which continue to this day, had been started by Michael Faraday in 1825. Porter firmly believed that Faraday would have appeared on television regularly if the technology had existed during his time.
I like to think that George Porter would approve of my Past Forward columns and my attempt to bring museum objects, history, and technology to an interested public. Porter was a firm proponent of scientists communicating their work to nonspecialists. He also thought that the scientific community should appreciate efforts to popularize their work. I suspect that Porter, who died in 2002, would have loved social media and the public debates it can inspire. I have no doubt he would be trending with his own hashtag.
Part of a continuing serieslooking at photographs of historical artifacts that embrace the boundless potential of technology.
An abridged version of this article appears in the March 2022 print issue as “The Nobelist’s First Laser.”
- What We Lose When We Lose Museums - IEEE Spectrum ›
- When Lasers Took to the Air to Measure the Earth - IEEE Spectrum ›
- 200 Years Ago, Faraday Invented the Electric Motor - IEEE Spectrum ›
Allison Marsh is a professor at the University of South Carolina and codirector of the university's Ann Johnson Institute for Science, Technology & Society. She combines her interests in engineering, history, and museum objects to write the Past Forward column, which tells the story of technology through historical artifacts.