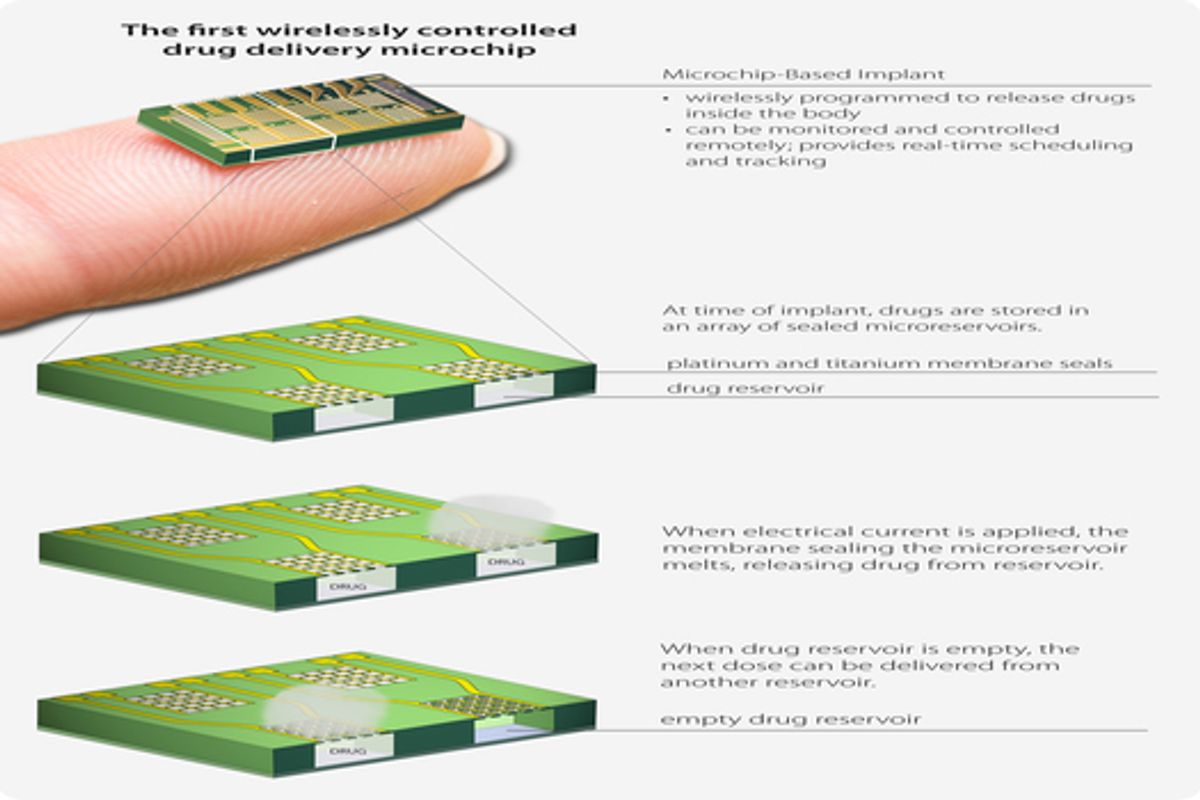
Researchers from the Boston-based medical technology company MicroCHIPS are presenting results this afternoon from their first clinical trial looking at the performance of an implanted drug delivery device. The results will also be published this afternoon in the AAAS journal, Science Translational Medicine. The device they tested is about as tall as a thumb drive, but slightly fatter. It contains 20 drug reservoirs, hermetically sealed with a metallic membrane which melts when exposed to an electrical current. When implanted under the skin around the waistline, the microchip can be wirelessly controlled by a doctor or programmed to release drugs on a set schedule.
During tests with animals, researchers in the company noticed that an envelope of fibrous tissue formed around the microchip. This first round of clinical trials was designed to test whether drugs can permeate through this build-up and to look at how effective the microchip is at getting drugs into the blood compared to normal injections.
For the trial, the reservoirs were filled with human parathyroid hormone fragment, a drug used to treat osteoporosis. Patients who use it undergo months of routine injections to slowly build up bone mass. As with any long-term injection schedule, there's a high risk that patients will get fed up and fall out of treatment. And this is the problem that MicroCHIPS wants to resolve. Seven women in the clinical trial agreed to have the chip implanted into their abdomens and left there for 4 months.
During the first two months, the researchers just left the device there, waiting for immune cells to gather. After giving enough time for a tissue capsule to grow, they started delivering drugs to the patients and taking blood samples. Although the drug seemed to make its way to the blood stream more slowly when released by the microchip, it reached nearly the same maximum concentration. More importantly, the results showed that the treatment was effective at initiating bone growth in the patients.
When the device was working, it seemed to work quite well. But it did occasionally malfunction. Of the 132 reservoirs that were programmed to open, only 116 did so perfectly. The other 16 either didn't open at all or opened only partially. The authors of the study claim that the malfunctions did not have an impact on the pharmacological results of the experiment. "There are 20 membranes per reservoir. We have demonstrated that when at least three quarters of them open, we achieve the same release profile as 100 percent," says Robert Farra, the president of MicroCHIPS and the lead author on the study. "We are working on our fabrication techniques to ensure 100 percent open. We have developed a system to identify whether or not a reservoir is empty, but we don't believe we will need to implement them given how successful our clinical trial was."*
If the technology holds up through further clinical trials, it will give patients an alternative to repeatedly poking themselves. But it's still not clear how good of a tradeoff this is. While the microchip only takes 30 minutes to implant, the procedure still requires cutting a slit into the skin. For their part, the study's participants reported little pain or discomfort from the implant. But they certainly didn't agree on whether they would go through the procedure again. My guess is it would have to replace more than a few months of injections to really be worth it. And this is the goal of the company as well. "We are working on a 365 dose device of the same size that will deliver daily doses for one year. Other disease therapies require every other day dosing which will result in a two-year longevity for the device," says Farra.*
Farra is also mindful of the recent security breeches that hackers have demonstrated with their own wirelessly controlled medical implants (such as this one) and he is working to secure the microchip as much as possible. "We are currently using a proprietary communication link and we will continue evolving this to ensure no unauthorized user is able to operate the implant," he says.*
*Content added on February 16, 2012 to include comments from Robert Farra.