THE INSTITUTE Those who have been infected by the coronavirus can display various symptoms. These include fever, difficulty breathing, and extreme fatigue, which can also be symptoms for several other illnesses. This can make it difficult for doctors to determine if a person is infected without a test, which sometimes can take days to find out the results. Many people suspected of being infected are quarantined at home, and they don’t have access to the specialized equipment doctors need to track their symptoms.
João Sanches and Hugo Silva are leading a team that has developed a mobile app that collects data about symptoms from users who either have been confirmed or suspected of having COVID-19, and monitors their health. The project is partially funded by the Portuguese National Science Foundation.
Sanches and Silva are IEEE senior members and bioengineering professors at the Instituto Superior Técnico in Lisbon. They are also researchers—Sanches at the Instituto Superior de Robótica and Silva at Instituto de Telecomunicações, also in Lisbon.
They are also officers in the IEEE Portugal Section’s IEEE Engineering in Medicine and Biology Society Chapter. Sanches is the vice chair and Silva is the secretary.
The Institute asked them about how the app works.
This interview has been edited and condensed for clarity.
What problem are you trying to solve?
Currently, people who are infected [or are suspected to be infected] with the coronavirus are either receiving treatment at a hospital or are monitored at home through periodic phone calls with doctors or are self-reporting [their] symptoms. Doctors do not have a way to automatically record and assess symptoms and track [the progression of the virus] for patients under quarantine.
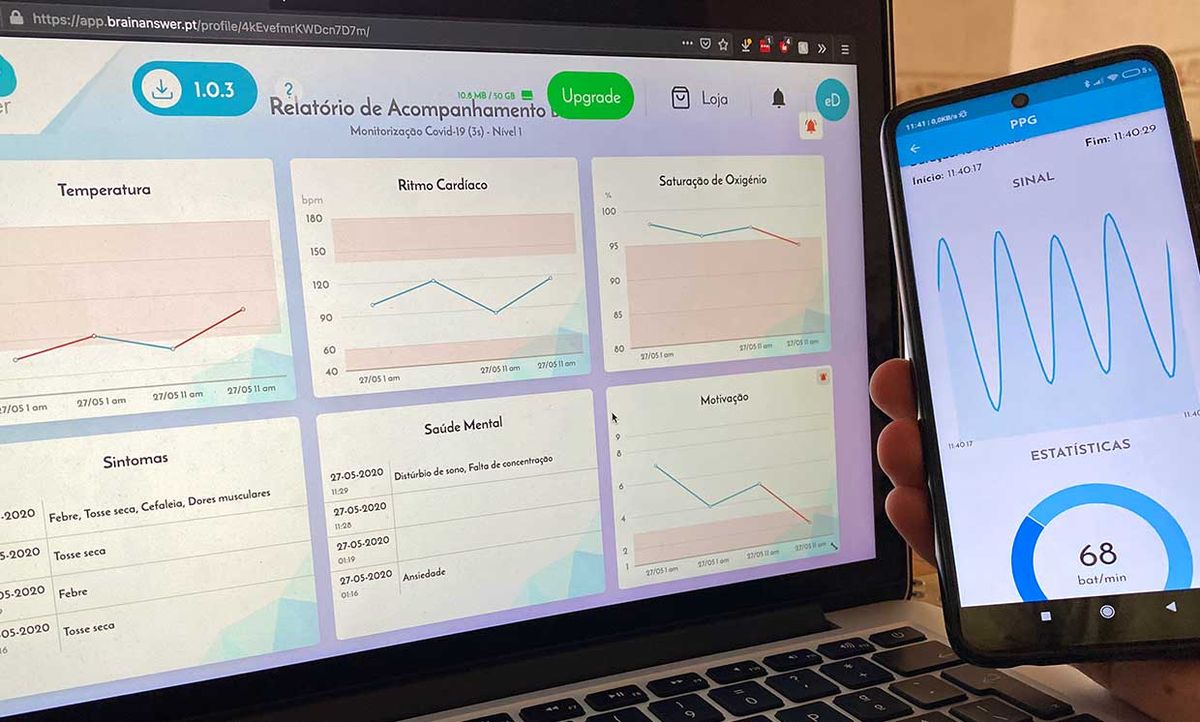
The mobile app, called e-CoVig, manages and visualizes clinical data of COVID-19 symptoms it collects from the user. It will acquire measurements such as pulse oximetry, temperature, heart rate, and respiration of patients, to help medical professionals better understand the symptoms of the virus.
What technologies are you using?
The system is comprised of a mobile phone as the primary interface coupled with a cloud-based eHealth platform for data collection.
E-CoVig is built on the cross-platform framework Flutter for the mobile app and uses BrainAnswer for cloud storage and analytics. BrainAnswer also serves as the Web-based patient management portal through which healthcare practitioners can review their patients’ data.
The mobile phone’s camera is used to record a video clip of the patient breathing and acquire data on the user’s heart rate by means of video photoplethysmography. [While filming the clip, the patient places his finger over the camera while the flash is turned on. The increase or decrease in arterial volume changes how much light from the flash is reflected by the finger and allows for the doctor to measure the patient's pulse.]
Patients can also record themselves using the microphone in order for the system to detect a cough and to periodically monitor respiratory activity. The user uses a headset with the microphone placed directly under the nostril.
Optical Character Recognition [a process that converts images of typed, handwritten, or printed text into machine-encoded text] is used to automate the process of recording the readings obtained from standard devices such as a thermometer, blood pressure monitor, and oxygen saturation level (SpO2) meter. The patient takes a photo of the device’s screen using the app and the measurement will be recorded.

Given that most of these devices only provide momentary readings [and some patients may not own these devices], we also developed a specialized wearable device that records SpO2, heart rate, and temperature data. The device, which is worn over the user’s ear for about a minute, is used for patients with enhanced risk of infection for COVID-19 or patients who have already been diagnosed. The data is sent to the patient’s profile on the app using Bluetooth.
Explain how your project works.
Patients undergoing e-CoVig monitoring are assigned a unique QR code that can be used across the different components of the system—mobile apps and website.
The mobile app is installed by the patient, caregiver, or healthcare professional, and serves as the primary data collection interface.
Qualitative assessment of the symptoms is performed by the patients periodically [every day or upon request of the healthcare practitioner] using an in-app measurement diary. The mobile app also issues reminders and enables the patient to access historical data. Patients fill out questionnaires daily, which enable the recording of other relevant clinical data by the patients to monitor if the symptoms are becoming more severe.
A quantitative assessment of physiological symptoms can also be performed by the patient every few hours with the wearable device. All the collected data is stored locally and synchronized with the BrainAnswer cloud when the smartphone is connected to the Internet.
The BrainAnswer platform provides a website with a user-friendly eHealth dashboard. On this platform, healthcare practitioners can review the data collected and receive alerts when abnormal parameters are detected or if measurements need to be taken. BrainAnswer can also extract and conduct a risk assessment of the monitored subjects following the guidelines set forth by the Trace COVID-19 task force—a team of doctors gathered by the Portuguese government and national healthcare service authorities. The group established the criteria for how to properly monitor patients diagnosed with COVID-19 remotely.
What challenges have you faced and how did you overcome them?
Finding a good balance between functionality and usability in order to devise a “human-friendly” solution has been a challenge. [We made sure that] healthcare practitioners [were a part of the project] since the beginning to ensure that we were creating a tool that would be effective and was made with doctors’ and patients’ needs in mind. [We are also] working with end-users in the field-testing stages to assess the usability of the different constituents of the solution.
Moving to the testing stage has also been a long process due to the current [COVID-19] mitigation measures in place, namely social distancing. The measure greatly restricts access to healthy and clinical subjects. We are starting to deploy features that patients and healthcare practitioners can use independently at home using their own devices and without physical contact.
What is the potential impact of the technology?
Diagnosed patients who do not require prolonged care in a hospital can be discharged to their homes [and] still be monitored remotely by healthcare practitioners. This allows the patient to avoid unnecessary trips to the hospital and potentially contaminating others. Patients who are unsure of their [infection] status can be provisionally observed at home, prior to visiting a clinical facility where they risk contaminating themselves (if they were not infected) or contaminating others (if they were infected but with low severity).
We believe the mobile app can greatly automate the interaction between patients, healthcare practitioners, and the national healthcare systems. It can reduce the risk of infection and help strengthen the monitoring process over time by increasing the number of symptoms the system can measure.
How close are you to the final product?
It will soon be implemented in a hospital environment and in a local health sciences school for clinical validation. Our goal is to quickly deploy e-CoVig for remote monitoring of individuals in assisted living homes, in isolation, and in quarantine.
How many people are involved, and how many IEEE members are involved?
There are 12 people working on the project. We work in various disciplines including biomedicine, electrical engineering, life sciences, software engineering, and design.
The team members are from several different Portuguese institutions in Lisbon such as the Hospital de Santa Maria, which is a leading National Health Service entity, and Instituto Superior de Robótica–ISR and Instituto de Telecomunicações-IT. They also include the health science school Escola Superior de Tecnologia da Saúde de Coimbra, and BrainAnswer.
How can other IEEE members get involved?
Anyone interested in being involved in the project can email João Sanches or Hugo Silva.