If I have a crusade on this blog, it is to see nanotechnology bring the battery up to snuff with all the high-tech gadgets they need to power.
I have covered how micrcoscopy tools are enabling us to pinpoint the reason batteries begin to fail. Recently I blogged on batteries that used nanowires to reduce their size down to that of a grain of salt.
The latest item I’ve come across in the way that nanotechnology is tackling the issue of improving the battery combines elements from the two blog entries I cited. The research claims to have produced the world’s smallest battery, which will help lead to better batteries in the future.
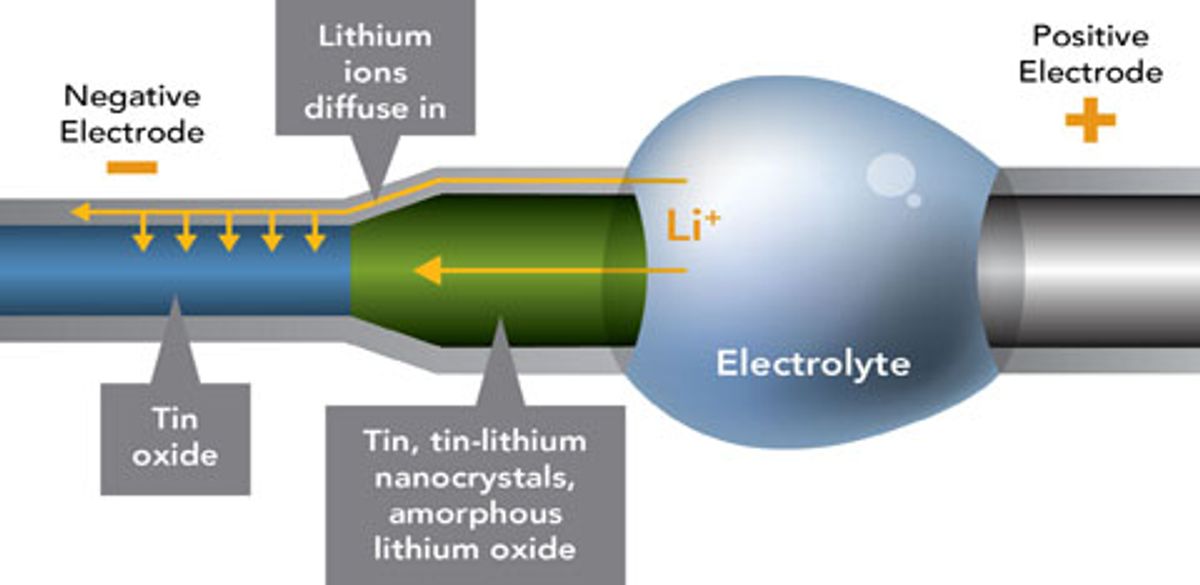
Researchers from Sandia National Laboratory have reported in the December 10th edition of Science of creating a battery so small that its anode consists of a single nanowire.
The lithium-based battery was created inside a transmission electron microscope (TEM) so we are not likely to see this battery powering your iPhone in the near future, but it does allow the researchers to see at an atomic scale resolution how batteries function to better understand their fundamental properties.
“What motivated our work," is that lithium ion batteries [LIB] have very important applications, but the low energy and power densities of current LIBs cannot meet the demand,” says Jianyu Huang. “To improve performance, we wanted to understand LIBs from the bottom up, and we thought in-situ TEM could bring new insights to the problem."
While nanomaterials are used in battery anodes, they are used in bulk rather than individually, a difference that Huang suggests makes it as difficult to observe their atomic structure as it would be to examine an individual tree among a forest.
The actual dimensions and parts of the battery consist “of a single tine oxide nanowire 100 nanometers in diameter and 10 micrometers long, a bulk lithium cobalt oxide cathode three millimeters long, and an ionic liquid electrolyte.”
One of the first unexpected phenomena the researchers observed was that the oxide nanowire nearly doubles in length in during charging, significantly more than its diameter increases. While this observation runs counter to the prevailing belief that diameter rather than length increases, it also could help avoid short circuits that may shorten battery life.
This observation could be significant but perhaps more significant was that the researchers found a way to use a liquid (the electrolyte) in the vacuum of a TEM.
“The methodology that we developed should stimulate extensive real-time studies of the microscopic processes in batteries and lead to a more complete understanding of the mechanisms governing battery performance and reliability," he said. "Our experiments also lay a foundation for in-situ studies of electrochemical reactions, and will have broad impact in energy storage, corrosion, electrodeposition and general chemical synthesis research field."
Dexter Johnson is a contributing editor at IEEE Spectrum, with a focus on nanotechnology.