The Boeing Dreamliner's problematic choice of a lithium-ion (Li-ion) battery designed around a cobalt oxide (CoO2) chemistry is making everyone a little nervous about Li-ion batteries in general. It harkens back to the mid-2000s when electronics giants apologetically had to recall the Li-ion batteries used in their laptop computers.
The CoO2 chemistry used in Li-Co-O2 batteries offers more power for its weight than the Li-Ti-O variety, but is less stable. However, if a better electrolyte solvent could be developed, much of that instability could be mitigated. (A commenter astutely discussed this topic in a response to a recent blog post.)
"To make a safer, lightweight battery, we need the design at the beginning to have safety in mind," said ORNL's leader researcher, Chengdu Liang, in a press release. "We started with a conventional material that is highly stable in a battery system—in particular one that is compatible with a lithium metal anode."
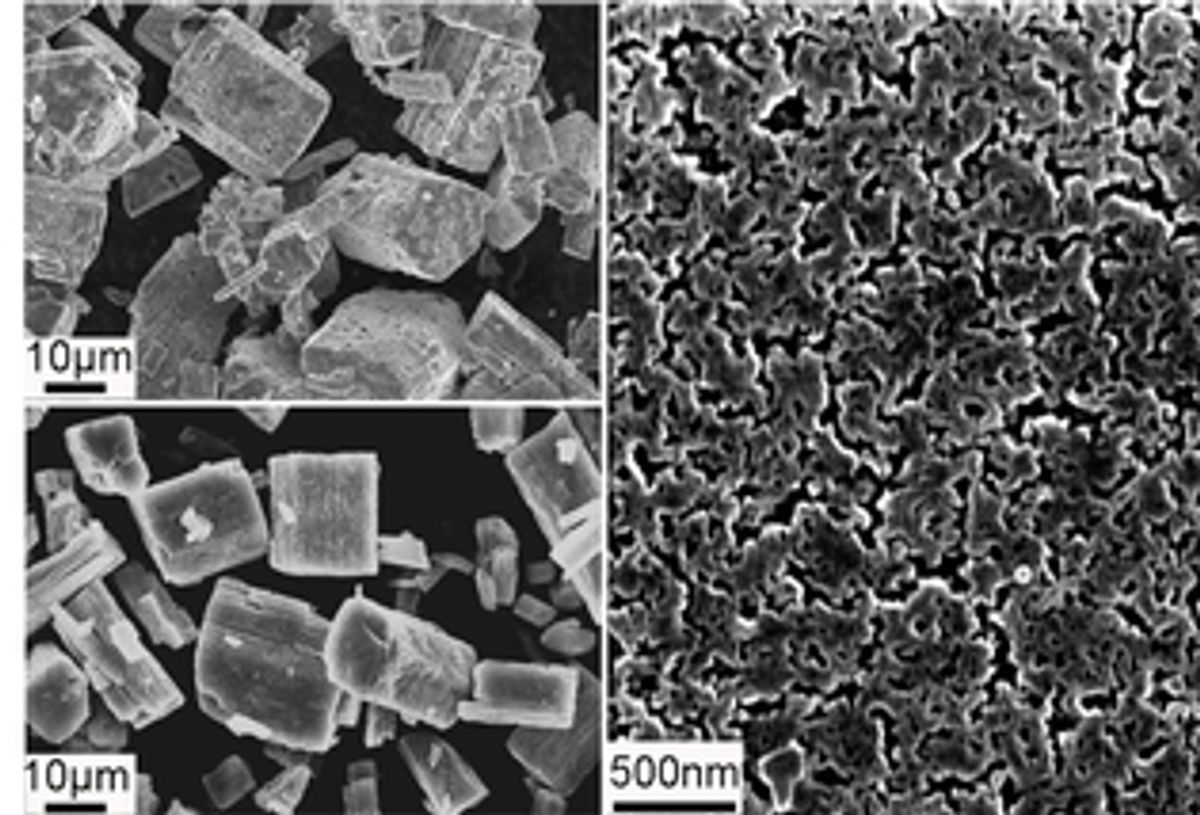
The research, which was published in the Journal of the American Chemical Society (“Anomalous High Ionic Conductivity of Nanoporous β-Li3PS4”), addressed one of the main drawbacks of using solid electrolytes: achieving a “high ionic conductivity and a broad electrochemical window.”
The material the researchers developed has a high-surface-to-bulk ratio that results from the material being broken down into nanometer dimensions.
"Think about it in terms of a big crystal of quartz vs. very fine beach sand," said coauthor Adam Rondinone in the press release. "You can have the same total volume of material, but it's broken up into very small particles that are packed together. It's made of the same atoms in roughly the same proportions, but at the nanoscale the structure is different. And now this solid material conducts lithium ions at a much greater rate than the original large crystal."
The researchers were able to demonstrate a three-orders-of-magnitude improvement over previous solid electrolytes when it comes to room-temperature lithium-ion conductivity. They believe that the ability to use a pure lithium metal anode with this solid electrolyte could lead to Li-ion batteries that are five to 10 times more powerful than batteries that use carbon-based anodes.
Liang adds: "Cycling highly reactive lithium metal in flammable organic electrolytes causes serious safety concerns. A solid electrolyte enables the lithium metal to cycle well, with highly enhanced safety."
While ORNL awaits its patent on this technology, it may be getting a frantic call from Boeing to see how quickly it can be brought to market.
Dexter Johnson is a contributing editor at IEEE Spectrum, with a focus on nanotechnology.