The landscape for two-dimensional materials has been getting a little crowded lately. We, of course, hear stories every day about graphene, but molybdenum disulfide has been getting a lot of interest and perhaps even edging ahead of graphene in transistor applications. Finally hexagonal boron nitride is gaining attention and rounding out this trilogy of 2-D materials that all promise to displace silicon in future electronics.
But another two-dimensional material has been in our midst for years and has largely gone unnoticed.
Researchers at Drexel University developed the 2-D material three years ago to little fanfare. But now they have discovered that the material has extraordinary energy storage capacity and could power flexible devices.
Michel W. Barsoum and Yury Gogotsi, both professors at Drexel’s college of engineering, dubbed the material an “MXene” because of its origin from the process of etching and exfoliating atomically thin layers of aluminum from layered carbides called “MAX phases.” [The M is for transition metal, the A for "A group" metal, and the X for carbon and/or nitrogen.]
Over the last three years, the engineers have been investigating applications for the materials. The research, which was published in the journal Science (“Cation Intercalation and High Volumetric Capacitance of Two-dimensional Titanium Carbide”), shows that MXenes store ions and molecules between layers of the material in a process known as intercalation.
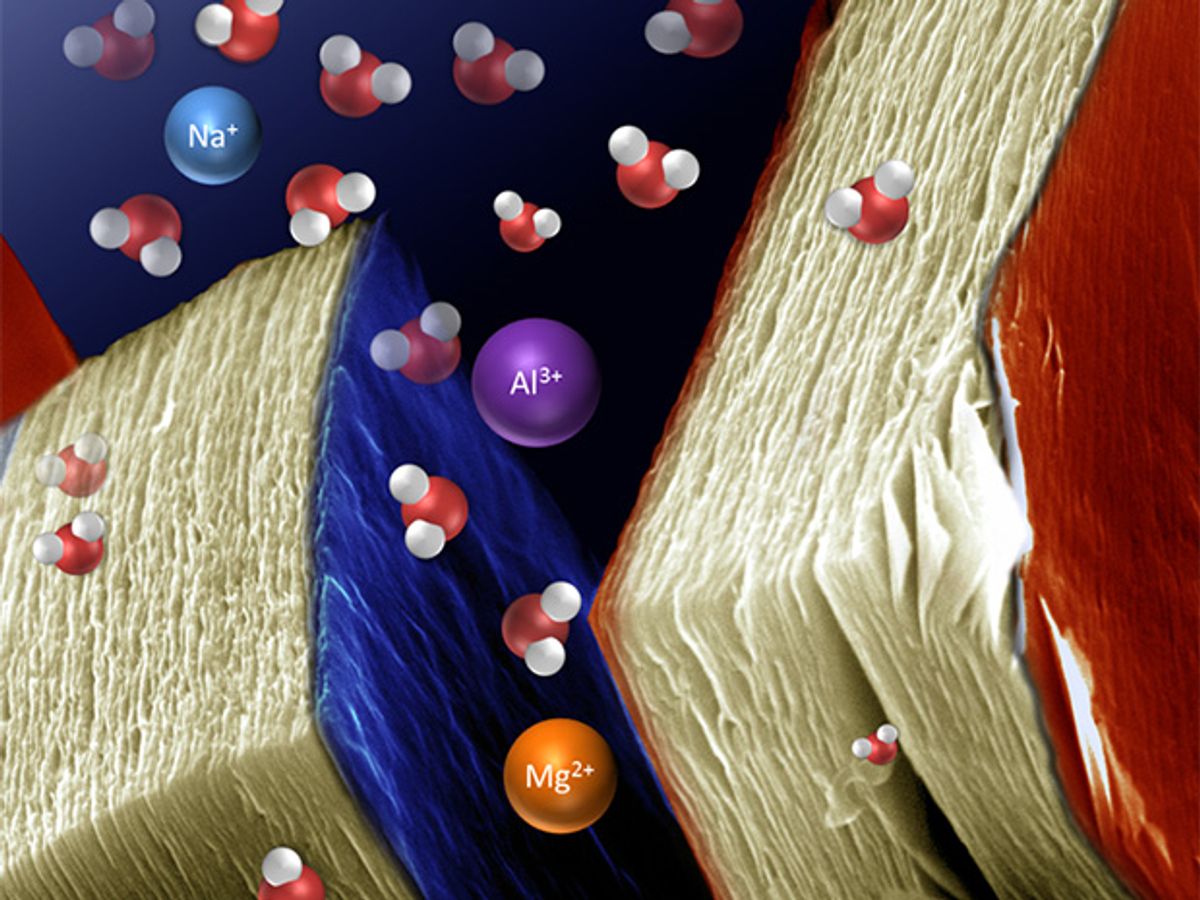
Intercalation is the inclusion of a molecule between two other molecules. The process is sometimes required in order to give two-dimensional materials their unique properties. In the Drexel research, they were able to place lithium ions between MXene sheets, making the materials good candidates for use in lithium-ion batteries.
“Currently, nine MXenes have been reported by our team, but there are likely many more that will be discovered—the MXene-and-ion combinations that have been tested to date are by no means an exhaustive demonstration of the material’s energy storage capabilities,” said Gogotsi, who is also director of the A.J. Drexel Nanotechnology Institute, in a press release. “So even the impressive capacitances that we are seeing here are probably not the highest possible values to be achieved using MXenes. Intercalation of magnesium and aluminum ions that we observed may also pave the way to development of new kinds of metal ion batteries.”
In addition to lithium, the researchers have been looking at intercalating ions of sodium, magnesium, potassium, ammonium, and aluminum in between the MXene sheets. In each case, the resulting material demonstrated a high capacity for energy storage, they said.
“Two-dimensional, titanium carbide MXene electrodes show excellent volumetric super capacitance of up to 350 F/cm3 due to intercalation of cations between its layers,” Barsoum said. “This capacity is significantly higher than what is currently possible with porous carbon electrodes. In other words, we can now store more energy in smaller volumes, an important consideration as mobile devices get smaller and require more energy”
- See more at: https://www.drexel.edu/now/news-media/releases/archive/2013/September/MXenes-Science/#sthash.PS22z2pL.dpuf“Two-dimensional, titanium carbide MXene electrodes show excellent volumetric super capacitance of up to 350 F/cm3 due to intercalation of cations between its layers,” Barsoum said. “This capacity is significantly higher than what is currently possible with porous carbon electrodes. In other words, we can now store more energy in smaller volumes, an important consideration as mobile devices get smaller and require more energy”
- See more at: https://www.drexel.edu/now/news-media/releases/archive/2013/September/MXenes-Science/#sthash.PS22z2pL.dpuf“Two-dimensional, titanium carbide MXene electrodes show excellent volumetric super capacitance of up to 350 F/cm3 due to intercalation of cations between its layers,” Barsoum said. “This capacity is significantly higher than what is currently possible with porous carbon electrodes. In other words, we can now store more energy in smaller volumes, an important consideration as mobile devices get smaller and require more energy”
- See more at: https://www.drexel.edu/now/news-media/releases/archive/2013/September/MXenes-Science/#sthash.PS22z2pL.dpuf“Two-dimensional, titanium carbide MXene electrodes show excellent volumetric super capacitance of up to 350 [farads per cubic centimeter] due to intercalation of cations between its layers,” Barsoum said in a press release. “This capacity is significantly higher than what is currently possible with porous carbon electrodes. In other words, we can now store more energy in smaller volumes, an important consideration as mobile devices get smaller and require more energy.”
Image: Drexel University
Dexter Johnson is a contributing editor at IEEE Spectrum, with a focus on nanotechnology.