The history of nanotechnology-based solutions for making fuel cells less expensive or more efficient has not really been what you would call a huge success. But a decade ago it seemed the focus on applying nanomaterials to areas such as improved catalysts for fuel cells was driven more by exploiting what the nanomaterials were good at rather than by what fuel cells needed to be more commercially viable.
Lately that dynamic has changed and nanotechnology has been finding itself more useful when it comes to cheap ways to isolate hydrogen—the high cost of which has been a key obstacle in reaching the so-called “hydrogen economy” where we can drive around in hydrogen-powered automobiles and power our mobile devices with fuel cells.
Three years ago, Angela Belcher at MIT mimicked the process of photosynthesis by developing a man-made virus that could effectively split water molecules into hydrogen and oxygen. Another team, at the University of California, also duplicated photosynthesis, but instead of exotic man-made viruses used a simpler nanowire-based material to cut the water molecules into its constituent parts.
The current state of the art for the artificial photosynthesis approach to isolating hydrogen may have been marked by HyperSolar Inc.’s announcement last year to commercialize a zero-carbon process for hydrogen gas production.
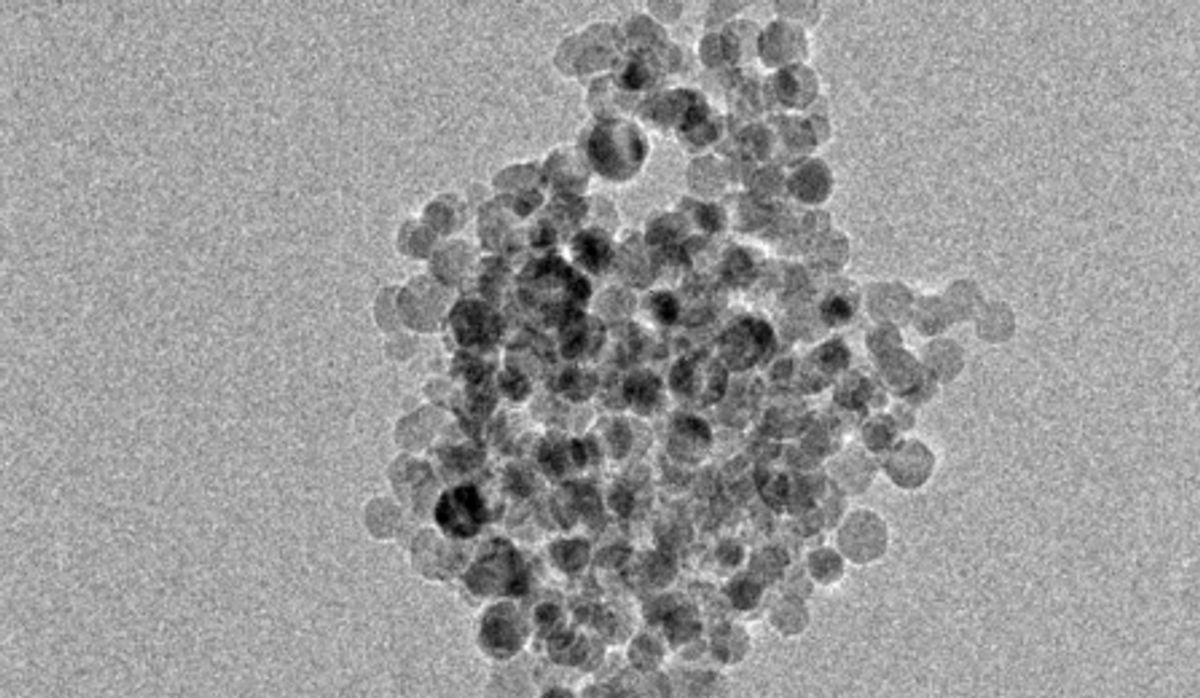
Not only did the reaction not require any light, but it also didn’t need heat or electricity. It was able to produce this reaction 150 times faster than when the same process is tried with silicon nanoparticles with dimensions of 100 nanometers. This translates into being able to produce the same amount of hydrogen gas in 1 minute with 10-nm silicon particles that it takes the 100-nm silicon particles 45 minutes to yield.
“It was previously unknown that we could generate hydrogen this rapidly from silicon, one of Earth’s most abundant elements,” said Folarin Erogbogbo, a research assistant professor at the University of Buffalo, in a press release.
This huge discrepancy in speed is the result of the particle geometries. When the larger particles are mixed with water they start forming into nonspherical structures that are not capable of reacting with the water in the way that the spherical 10-nm silicon particles do.
The researchers do concede that producing the 10-nm silicon balls is energy intensive, so using this method for the hydrogen economy may not be economical. However, in certain circumstances where water is readily available, a small package of these nanoscale silicon particles could be useful in powering small portable devices.
“Perhaps instead of taking a gasoline or diesel generator and fuel tanks or large battery packs with me to the campsite (civilian or military) where water is available, I take a hydrogen fuel cell (much smaller and lighter than the generator) and some plastic cartridges of silicon nanopowder mixed with an activator,” said researcher Mark T. Swihart, UB professor of chemical and biological engineering and director of the university’s Strategic Strength in Integrated Nanostructured Systems. “Then I can power my satellite radio and telephone, GPS, laptop, lighting, etc. If I time things right, I might even be able to use excess heat generated from the reaction to warm up some water and make tea.”
It is encouraging when researchers recognize the limitations of their research and sort out how they could still serve a useful purpose.
Image: Swihart Research Group, University at Buffalo
Dexter Johnson is a contributing editor at IEEE Spectrum, with a focus on nanotechnology.